Identification of a selective pyruvate dehydrogenase kinase 1 (PDHK1) chemical probe by virtual screening
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
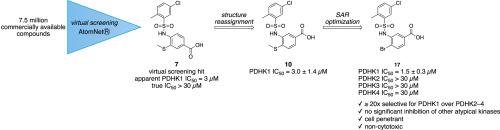
PDHK1 is a non-canonical Ser/Thr kinase that negatively regulates the pyruvate dehydrogenase complex (PDC), restricting entry of acetyl-CoA into the tricarboxylic acid (TCA) cycle and downregulating oxidative phosphorylation. In many glycolytic tumors, PDHK1 is overexpressed to suppress activity of the PDC and cause a shift in metabolism toward an increased reliance on glycolysis (the Warburg effect). Genetic studies have shown that knockdown or knockout of PDHK1 reverts this phenotype and inhibits tumor growth in vitro and in vivo, but chemical tools to pharmacologically validate and build upon these data are lacking. We used AtomNet®, a deep convolutional neural network bioactivity predictor, to identify compound 7 as a potential inhibitor of PDHK1. During the process of hit validation, the active species was determined to be isomeric compound 10. Structure-activity studies based on 10 identified 17 as a low μM inhibitor of PDHK1 (IC50 = 1.5 ± 0.3 μM) that is selective against the other PDHK isoforms in both biochemical and cell-based assays. In A549 epithelial lung carcinoma cells, compound 17 inhibits phosphorylation of PDC E1α Ser232, a site that is specifically phosphorylated only by PDHK1, while minimally suppressing phosphorylation of Ser293, a site that is phosphorylated by all four PDHK isoforms. Altogether, these data identify 17 as a selective PDHK1 chemical probe useful for biochemical and cell-based studies.

选择性丙酮酸脱氢酶激酶1 (PDHK1)化学探针的虚拟筛选鉴定
PDHK1是一种非规范的丝氨酸/苏氨酸激酶,它负调控丙酮酸脱氢酶复合物(PDC),限制乙酰辅酶a进入三羧酸(TCA)循环并下调氧化磷酸化。在许多糖酵解性肿瘤中,PDHK1过表达抑制PDC活性,导致代谢向糖酵解依赖性增加转变(Warburg效应)。遗传学研究表明,敲低或敲除PDHK1可以恢复这种表型,并在体外和体内抑制肿瘤生长,但缺乏化学工具来药理学验证和建立这些数据。我们使用深度卷积神经网络生物活性预测器AtomNet®来识别化合物7作为PDHK1的潜在抑制剂。在命中验证过程中,确定活性物质为同分异构体化合物10。基于10的结构活性研究发现17是PDHK1的低μM抑制剂(IC50 = 1.5±0.3 μM),在生化和细胞实验中对其他PDHK亚型具有选择性。在A549上皮性肺癌细胞中,化合物17抑制PDC E1α Ser232的磷酸化,这是一个仅被PDHK1特异性磷酸化的位点,而最低限度地抑制Ser293的磷酸化,Ser293是一个被PDHK所有四种亚型磷酸化的位点。总之,这些数据确定17是一种选择性的PDHK1化学探针,可用于生化和基于细胞的研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


