Mechanistic Insights into Potassium-Assistant Thermal-Catalytic Oxidation of Soot over Single-Crystalline SrTiO3 Nanotubes with Ordered Mesopores
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Soot catalytic combustion using single-crystalline perovskite-type materials holds great promise as an efficient non-noble metal catalyst, with K+-modified SrTiO3 emerging as one of the most desirable candidates. However, balancing the crystallinity and an optimized pore structure and revealing the mechanism underlying the K+ action remain challenges. Herein, by the electrospinning technique, we successfully self-assembled the K-doped single-crystalline SrTi0.95Al0.05O3 nanotubular webs with ordered mesopores. The good crystallinity and mesoporous structures contribute to the enhanced catalytic performance with desirable stability. Based on comprehensive characterizations and density functional theory (DFT) calculations, K+ ions effectively accumulate defect charges, facilitating the generation of additional oxygen vacancies and expediting oxygen activation during the reaction. Additionally, the presence of K+ ions prefers to preserve O2 bond integrity during activation, significantly increasing NO adsorption capacity. Utilizing KNO3 as the medium, K+ effectively facilitates the storage and subsequent release of active oxygen species, leading to the promised catalytic performance (T50 = 368 °C, Ea = 64.97 kJ mol–1, TOFK = 0.017 h–1). This study provides mechanistic insights into developing advanced materials for thermal catalytic heterogeneous reactions.
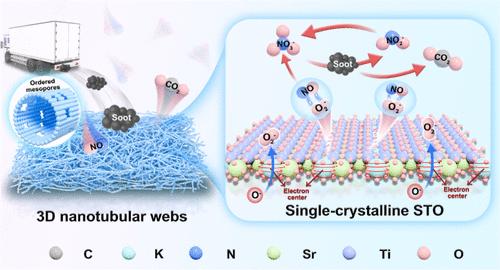
煤烟在有序介孔单晶SrTiO3纳米管上的钾辅助热催化氧化机理研究
单晶钙钛矿型材料的烟尘催化燃烧作为一种高效的非贵金属催化剂具有很大的前景,其中K+改性SrTiO3成为最理想的候选者之一。然而,平衡结晶度和优化孔隙结构以及揭示K+作用的机制仍然是一个挑战。通过静电纺丝技术,我们成功地自组装了具有有序介孔的k掺杂单晶SrTi0.95Al0.05O3纳米管网。良好的结晶度和介孔结构有助于提高催化性能,并具有理想的稳定性。基于综合表征和密度泛函理论(DFT)计算,K+离子有效地积累缺陷电荷,促进额外氧空位的产生,加速反应过程中的氧活化。此外,在活化过程中,K+离子的存在更倾向于保持O2键的完整性,显著提高了NO的吸附能力。以KNO3为介质,K+有效地促进了活性氧的储存和后续释放,从而获得了预期的催化性能(T50 = 368°C, Ea = 64.97 kJ mol-1, TOFK = 0.017 h-1)。该研究为开发用于热催化非均相反应的先进材料提供了机理见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


