Nonspherical Particle Stabilized Emulsions Formed through Destabilization and Arrested Coalescence
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
To form nonspherical emulsion droplets, the interfacial tension driving droplet sphericity must be overcome. This can be achieved through interfacial particle jamming; however, careful control of particle coverage is required. In this work, we present a scalable novel batch process to form nonspherical particle-stabilized emulsions. This is achieved by concurrently forming interfacially active particles and drastically accelerating emulsion destabilization through addition of electrolyte. To achieve this, surfactant-stabilized oil-in-water emulsions in the presence of dopamine were first produced. These emulsions were then treated with tris(hydroxymethyl)aminomethane hydrochloride buffer to both simultaneously initiate polymerization of dopamine in the emulsion continuous phase and reduce the Debye length of the system, thus accelerating droplet coalescence while forming surface-active particles. The concentration of buffer and imposed shear was then systematically varied, and the behavior at the interface was studied using pendent drop tensiometry and interfacial shear rheology. It was found that polydopamine nanoparticles formed in the emulsion continuous phase adsorbed to the reducing interface during coalescence, resulting in anisotropic droplets formed via arrested coalescence. Greater shear rates resulted in accelerated coalescence and formation of secondary droplets, whereas lower shear rates resulted in thicker interfacial films. The efficacy of this method was further demonstrated with a second system consisting of sodium dodecyl sulfate as the surfactant and polypyrrole particles, which also resulted in nonspherical droplets for optimized conditions.
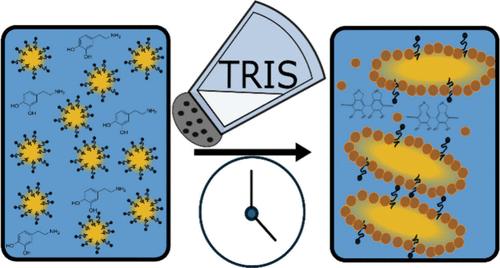
非球形颗粒稳定乳状液通过失稳和聚结阻滞形成
为了形成非球形乳化液液滴,必须克服驱动液滴球形的界面张力。这可以通过界面粒子干扰来实现;但是,需要仔细控制颗粒覆盖范围。在这项工作中,我们提出了一种可扩展的新批量工艺来形成非球形颗粒稳定乳液。这是通过同时形成界面活性颗粒和通过添加电解质急剧加速乳液不稳定来实现的。为了实现这一目标,首先在多巴胺存在下生产了表面活性剂稳定的水包油乳液。然后用三(羟甲基)氨基甲烷盐酸缓冲液处理这些乳状液,同时在乳状液连续相中引发多巴胺的聚合,并减少体系的德拜长度,从而加速液滴聚并形成表面活性颗粒。然后系统地改变缓冲液和施加剪切的浓度,并使用垂滴张力计和界面剪切流变学研究了界面上的行为。研究发现,聚多巴胺纳米颗粒在聚结过程中吸附在还原界面上,导致聚结阻滞形成各向异性的微滴。剪切速率越高,二次液滴的聚结和形成越快,剪切速率越低,界面膜越厚。以十二烷基硫酸钠为表面活性剂,用聚吡咯颗粒组成的第二体系进一步验证了该方法的有效性,该体系在优化条件下也得到了非球形液滴。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


