Low density (100-x)Li3BS3-xLiI solid-state electrolyte with ultra-long cycling stability for solid-state battery
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
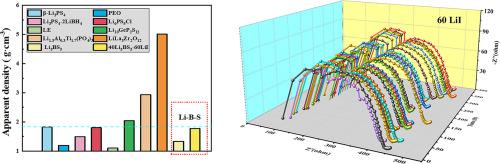
Thioborate solid-state electrolytes are considered to be ideal solid-state electrolytes (SSEs) due to their high theoretical ionic conductivity, wide electrochemical stability window, abundant raw materials, and low density. However, due to the demanding synthesis conditions, thioborate SSEs have yet to be investigated systematically and their electrochemical properties remain largely underdeveloped. In this work, a series of (100-x)Li3BS3-xLiI SSEs was prepared from Li2S, B, S, and LiI by melt quenching, and the effects of I doping on the electrochemical properties of Li3BS3 SSEs were investigated systematically. The as-prepared 40Li3BS3-60LiI SSEs exhibits an excellent ionic conductivity of 0.42 mS cm−1 and a low apparent density of 1.334 g cm−3 at 25°C. The ultra-high dissolution capacity up to 60% mol ratio of LiI in Li3BS3 enhances interfacial stability, which contributes to a long cycling life of 330 hours at 0.1 mA cm−2 without significant interfacial degradation. These results suggest that Li3BS3-LiI SSEs with high ionic conductivity and excellent interfacial stability could facilitate the development of all-solid-state batteries with high energy density, long-term cycling stability, and low cost.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


