The potential range switches between anion and cation-driven actuation: comparative study of three actuating polypyrrole materials in organic media
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
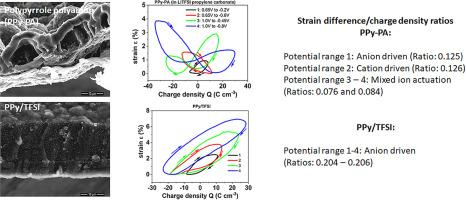
Three different polypyrrole (PPy) material films: polypyrrole-polyanions (PPy-PA) generated by simultaneous electropolymerization, polypyrrole-dodecylbenzenesulfonate (PPy/DBS) and Polypyrrole-bis(trifluoromethylsulfonyl)imide (PPy/TFSI), were electrogenerated and then characterized as linear actuators (strain evolution) in propylene carbonate solutions by cyclic voltammetry and square potential waves. Four different potential ranges (1, 0.65V to -0.25V; 2, 0.65V to -0.6V, 3, 1.0V to -0.45V and 4, 1.0V to -0.8V) were studied. Oxidation and reduction charges were in balance whatever the material or the applied potential range. The PPy-PA actuator gives a mainly anion-driven linear actuation in the potential range 1 shifting to cation-driven actuation in the potential range 2, having mixed ion actuation in the potential ranges 3-4. This fast experimental shift of the actuation mechanism open a new way for both, applications and chemical kinetic study of those biomimetic reactions. The PPy/DBS actuator gives a mainly anion-driven actuation and some minor mixed-ion actuation, while PPy/TFSI showed consistent anion-driven in the four studied potential ranges. The evolution of the strain/specific charge ratio (actuator efficiency) can allow, in absence of parallel irreversible reactions, a quantitative description of the mixed actuation here and of the different Faradaic actuators in the future. The three studied materials give Faradaic actuators (charge/strain linear dependency) and closed loops strain/charge responses (without creeping) allowing a good actuation control and low degradation. Further material characterization such as scanning electron microscopy (SEM), Fourier-transform infrared (FTIR), energy dispersive X-ray spectroscopy (EDX), electronic conductivity or ionic diffusion coefficient determinations are conducted.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


