Adipocyte-derived ferroptotic signaling mitigates obesity
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Ferroptosis is characterized as an iron-dependent and lipophilic form of cell death. However, it remains unclear what role ferroptosis has in adipose tissue function and activity. Here, we find a lower ferroptotic signature in the adipose tissue of individuals and mice with obesity. We further find that activation of ferroptotic signaling by a non-lethal dose of ferroptosis agonists significantly reduces lipid accumulation in primary adipocytes and high-fat diet (HFD)-fed mice. Notably, adipocyte-specific overexpression of acyl-coenzyme A synthetase long-chain family member 4 (Acsl4) or deletion of ferritin heavy chain (Fth) protects mice from HFD-induced adipose expansion and metabolic disorders via activation of ferroptotic signaling. Mechanistically, we find that 5,15-dihydroxyeicosatetraenoic acid (5,15-DiHETE) activates ferroptotic signaling, resulting in the degradation of hypoxia-inducible factor-1α (HIF1α), thereby derepressing a thermogenic program regulated by the c-Myc-peroxisome proliferator-activated receptor gamma coactivator-1 beta (Pgc1β) pathway. Our findings suggest that activating ferroptosis signaling in adipose tissues might help to prevent and treat obesity and its related metabolic disorders.
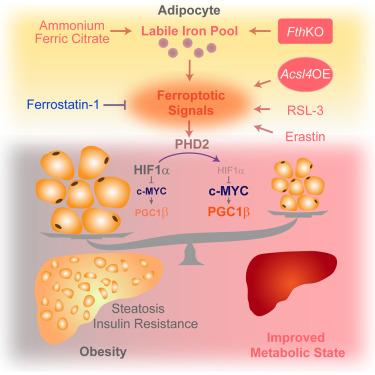
脂肪细胞衍生的嗜铁信号传导可减轻肥胖
铁下垂的特征是一种铁依赖性和亲脂性的细胞死亡形式。然而,尚不清楚铁下垂在脂肪组织功能和活性中的作用。在这里,我们发现肥胖个体和小鼠的脂肪组织中有较低的铁下垂特征。我们进一步发现,通过非致死剂量的铁致凋亡激动剂激活铁致凋亡信号可以显著减少原代脂肪细胞和高脂肪饮食(HFD)喂养小鼠的脂质积累。值得注意的是,脂肪细胞特异性的酰基辅酶A合成酶长链家族成员4 (Acsl4)的过表达或铁蛋白重链(Fth)的缺失通过激活铁致凋亡信号保护小鼠免受hfd诱导的脂肪扩张和代谢紊乱。在机制上,我们发现5,15-二羟基二碳四烯酸(5,15- dihete)激活铁致凋亡信号,导致缺氧诱导因子-1α (HIF1α)的降解,从而抑制由c- myc -过氧化物酶体增殖体激活受体γ辅助激活因子-1 β (Pgc1β)途径调节的产热程序。我们的研究结果表明,激活脂肪组织中的铁下垂信号可能有助于预防和治疗肥胖及其相关的代谢紊乱。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
文献相关原料
公司名称
产品信息
索莱宝
Collagenase IV
索莱宝
Triglyceride Assay kit
索莱宝
Collagenase IV
Sigma
Tyloxapol
Sigma
Triton X-100
Sigma
Collagenase II
Sigma
Dispase II
Sigma
3,3',5-Triiodo-L-thyronine sodium salt
Sigma
Dexamethasone
Sigma
Indomethacin
Sigma
DAPI
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


