Disrupted methionine cycle triggers muscle atrophy in cancer cachexia through epigenetic regulation of REDD1
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
The essential amino acid methionine plays a pivotal role in one-carbon metabolism, facilitating the production of S-adenosylmethionine (SAM), a critical supplier for DNA methylation and thereby a modulator of gene expression. Here, we report that the methionine cycle is disrupted in skeletal muscle during cancer cachexia, leading to endoplasmic reticulum stress and DNA hypomethylation-induced expression of the DNA damage inducible transcript 4 (Ddit4) gene, encoding the regulated in development and DNA damage response 1 (REDD1) protein. Targeting DNA methylation by depletion or pharmacological inhibition of DNA methyltransferase 3A (DNMT3A) exacerbates cachexia, while restoring DNMT3A expression or REDD1 knockout alleviates cancer cachexia-induced skeletal muscle atrophy in mice. Methionine supplementation restores DNA methylation of the Ddit4 promoter in a DNMT3A-dependent manner, thereby inhibiting activating transcription factor 4 (ATF4)-mediated Ddit4 transcription. Thus, with the identification of the methionine/SAM-DNMT3A/DNA hypomethylation-Ddit4/REDD1 axis, our study provides molecular insights into an epigenetic mechanism underlying cancer cachexia, and it suggests nutrient supplementation as a promising therapeutic strategy to prevent or reverse cachectic muscle atrophy.
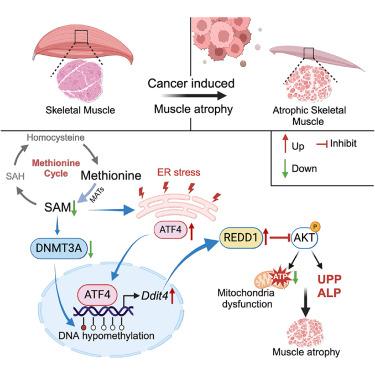
蛋氨酸周期中断通过REDD1的表观遗传调控引发癌症恶病质中的肌肉萎缩
必需氨基酸蛋氨酸在单碳代谢中起关键作用,促进s -腺苷蛋氨酸(SAM)的产生,SAM是DNA甲基化的关键供应商,因此是基因表达的调节剂。在这里,我们报道了在癌症恶病质期间骨骼肌中的蛋氨酸循环被破坏,导致内质网应激和DNA低甲基化诱导的DNA损伤诱导转录物4 (Ddit4)基因的表达,该基因编码发育和DNA损伤反应1 (REDD1)蛋白。通过消耗或药物抑制DNA甲基化酶3A (DNMT3A)来靶向DNA甲基化会加剧恶病质,而恢复DNMT3A表达或敲除REDD1可减轻小鼠癌症恶病质诱导的骨骼肌萎缩。补充蛋氨酸以依赖dnmt3a的方式恢复Ddit4启动子的DNA甲基化,从而抑制转录因子4 (ATF4)介导的Ddit4转录的激活。因此,通过鉴定甲硫氨酸/SAM-DNMT3A/DNA低甲基化- ddit4 /REDD1轴,我们的研究为癌症恶病质的表观遗传机制提供了分子见解,并表明营养补充是预防或逆转恶病质肌萎缩的一种有希望的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


