Single-cell analysis of bidirectional reprogramming between early embryonic states identify mechanisms of differential lineage plasticities in mice
IF 10.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Two distinct lineages, pluripotent epiblast (EPI) and primitive (extra-embryonic) endoderm (PrE), arise from common inner cell mass (ICM) progenitors in mammalian embryos. To study how these sister identities are forged, we leveraged mouse embryonic stem (ES) cells and extra-embryonic endoderm (XEN) stem cells—in vitro counterparts of the EPI and PrE. Bidirectional reprogramming between ES and XEN coupled with single-cell RNA and ATAC-seq analyses showed distinct rates, efficiencies, and trajectories of state conversions, identifying drivers and roadblocks of reciprocal conversions. While GATA4-mediated ES-to-iXEN conversion was rapid and nearly deterministic, OCT4-, KLF4-, and SOX2-induced XEN-to-induced pluripotent stem (iPS) reprogramming progressed with diminished efficiency and kinetics. A dominant PrE transcriptional program, safeguarded by GATA4, alongside elevated chromatin accessibility and reduced DNA methylation of the EPI underscored the differential plasticities of the two states. Mapping in vitro to embryo trajectories tracked reprogramming cells in either direction along EPI and PrE in vivo states, without transitioning through the ICM.
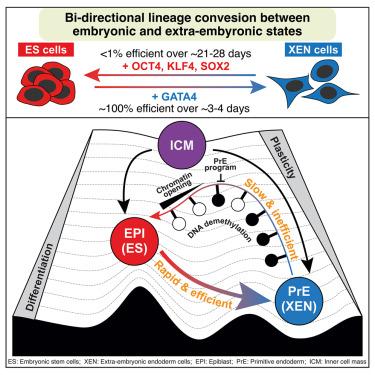
单细胞分析双向重编程之间的早期胚胎状态确定机制的差异谱系可塑性在小鼠
多能外胚层(EPI)和原始(胚胎外)内胚层(PrE)这两种不同的细胞系起源于哺乳动物胚胎中共同的内细胞团(ICM)祖细胞。为了研究这些姐妹身份是如何形成的,我们利用了小鼠胚胎干细胞(ES)和胚胎外内胚层(XEN)干细胞——EPI和PrE的体外对应体。ES和XEN之间的双向重编程结合单细胞RNA和ATAC-seq分析显示了不同的状态转换速率、效率和轨迹,确定了相互转换的驱动因素和障碍。虽然gata4介导的ES-to-iXEN转换是快速且几乎确定的,但OCT4-, KLF4-和sox2诱导的XEN-to-induced plurpotent stem (iPS)重编程的效率和动力学降低。一个由GATA4保护的显性前转录程序,以及染色质可及性的升高和EPI DNA甲基化的降低,强调了两种状态的不同可塑性。在体外到胚胎的轨迹映射中,沿着EPI和PrE的两个方向跟踪了重编程细胞在体内的状态,而没有通过ICM过渡。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


