Intelligent Microneedles Patch with Wireless Self-Sensing and Anti-Infective Actions
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
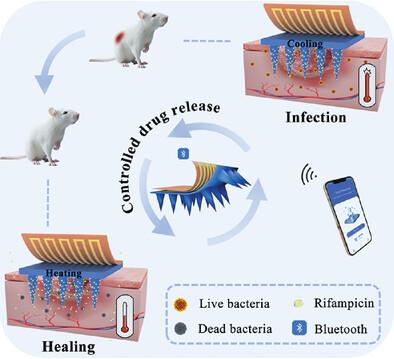
Traditional microneedle (MN) technology offers unique advantages in treating wound infections; however, its single-function design lacks the capability for real-time monitoring of wound conditions, often resulting in uncontrolled drug release. Herein, an anti-infective and intelligent MN patch (SP-CSMN) integrating three functional modules is developed, including temperature monitoring, Bluetooth wireless communication, and responsive drug release. The patch employed chitosan (CS) as a porous substrate, filled with temperature-sensitive poly(N-isopropylacrylamide) (PNIPAM) to encapsulate and release the antibiotic rifampicin. With the integrated sensing chip, SP-CSMN enabled continuous temperature monitoring and real-time feedback via smartphone Bluetooth communication. When the wound temperature exceeds 36.5 °C for 6 h, the system can automatically identify the infection occurrence and activate the heating module to trigger PNIPAM contraction, triggering rifampicin release. This self-sensing and intelligent release cycles can repeat throughout its life-cycle. The SP-CSMN demonstrated precisely temperature-induced drug release and enhanced antibacterial activities against Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) in vitro. Furthermore, it sensitively monitored wound temperature changes in infected mice and significantly accelerated wound healing via controlled drug delivery. This advanced MN system offers a promising solution for efficient management of bacterial wound infections.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


