MICROSCAFS® for minocycline elimination from water and real wastewater: Porosity and TiO2 nanoparticles effect
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
We developed and characterized a photocatalytic system utilizing sol–gel synthesized silica-titania microspheres (MICROSCAFS®) with varying interconnected porosities, functioning as a photocatalyst by itself, but also as a support for TiO2 nanoparticles. This system was employed to remove the antibiotic minocycline from water, including real wastewater from a minocycline production plant, either in batch or flow photoreactor setups. We evaluated the pore size of MICROSCAFS® and found that the combination of medium-sized macropores together with the largest specific surface area (P0/HT) provided the highest adsorption capacity, achieving 13 % in the dark, attributed to the interaction between the positively charged antibiotic and the negatively charged MICROSCAFS®. Photocatalytic activity varied among samples, with P0/HT achieving the highest photodegradation of 73 % after 300 min of solar irradiation in a flow reactor. The immobilization of P25 TiO2 nanoparticles (P0/HT+P25) optimized the removal, achieving 100 % degradation within 270 min. LC-HRMS/MS analysis identified specific by-products, which were fewer and less toxic than minocycline, and DFT and in-silico toxicity studies corroborate these findings. Scavenger tests highlighted the role of •OH, h+, and •O2–, with •O2– being the primary contributor. This photocatalyst, tested with real wastewater from a pharmaceutical industry, demonstrated effective environmental remediation without the need for expensive separation steps. Flow reactor experiments and a modeling study underscored its potential for continuous pollutant degradation in practical applications.
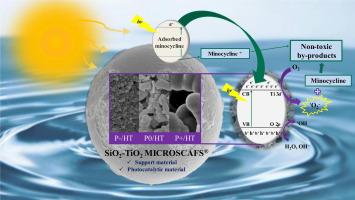
MICROSCAFS®用于从水和实际废水中消除米诺环素:孔隙率和TiO2纳米颗粒效应
我们开发并表征了一种光催化系统,该系统利用溶胶-凝胶合成的具有不同互连孔隙率的二氧化硅-二氧化钛微球(MICROSCAFS®),其本身既可以作为光催化剂,也可以作为TiO2纳米颗粒的载体。该系统用于去除水中的抗生素二甲胺四环素,包括来自二甲胺四环素生产厂的实际废水,无论是批处理还是流动光反应器设置。我们评估了MICROSCAFS®的孔径,发现中型大孔与最大比表面积(P0/HT)的组合提供了最高的吸附容量,在黑暗中达到13 %,这归功于带正电的抗生素与带负电的MICROSCAFS®之间的相互作用。不同样品的光催化活性不同,在流动反应器中,经过300 min的太阳照射后,P0/HT的光降解率最高,达到73 %。P25 TiO2纳米颗粒(P0/HT+P25)的固定化优化了去除效果,在270 min内达到100% %的降解率。LC-HRMS/MS分析确定了特定的副产物,比米诺环素更少,毒性更小,DFT和硅毒性研究证实了这些发现。清道夫测试强调了•OH、h+和•O2 -的作用,其中•O2 -是主要的贡献者。这种光催化剂在制药工业的实际废水中进行了测试,证明了它可以有效地修复环境,而不需要昂贵的分离步骤。流动反应器实验和模型研究强调了其在实际应用中持续降解污染物的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


