Origin of Stereoselectivity in Ring Opening Metathesis Polymerization with Cationic Molybdenum Imido Alkylidene CAAC Complexes
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
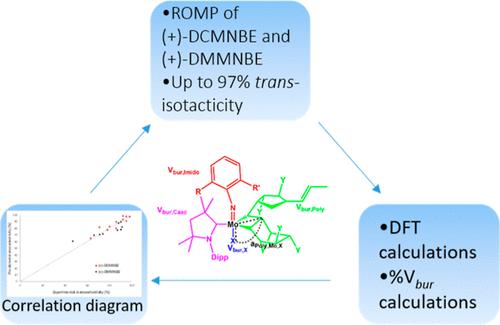
Stereoselective ring opening metathesis polymerization (ROMP) of enantiomerically pure 2,3-dicarbomethoxynorborn-5-ene ((+)-DCMNBE) was accomplished by the action of cationic tetra- and pentacoordinated molybdenum imido alkylidene cyclic alkyl amino carbene (CAAC) complexes that are chiral at molybdenum. The same catalysts were also utilized to perform the ROMP of 2,3-dimethoxymethylnorborn-5-ene ((+)-DMMNBE). All complexes were moderately to highly active and showed high trans-isoselectivity, offering up to 97% trans-isotactic (it) repeat units. In all cases, tetracoordinated complexes were the active species, resulting in pentacoordinated transition states. A theoretical model was elaborated using the buried volume (% Vbur) values of all ligands from single-crystal X-ray analysis together with the structures of the density functional theory (DFT) generated molybdacyclobutane intermediates. The model demonstrates the steric effects of all ligands at molybdenum on the trans-isoselectivity of the reaction, as predicted by the turnstile mechanism, and includes a positive correlation between the bulky CAAC ligand with high values of % Vbur of the other ligands and a high trans-isoselectivity. It was also successfully extended to molybdenum imido alkylidene N-heterocyclic carbene (NHC) complexes, proved to be of sufficient accuracy with a root mean squared error (RMSE) of 6.19% and was verified by Monte Carlo cross-validation (MCCV).
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


