ABO4 as an Active Catalyst Structure for Direct Partial CH4 Oxidation as Identified through Screening of Supported Catalysts
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
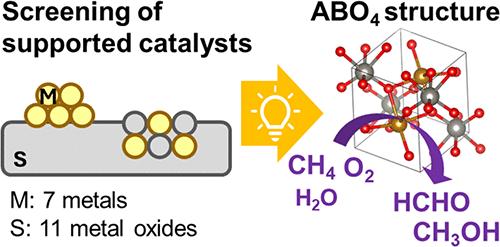
In the present study, 76 different metal-oxide-supported-transition-metal catalysts were prepared using 11 different metal oxides (MgO, Al2O3, SiO2, TiO2, V2O5, ZrO2, Nb2O5, MoO3, Ta2O5, WO3, and La2O3) and seven 3d metals (V, Mn, Fe, Co, Ni, Cu, and Zn). The 76 supported catalysts, along with 11 single metal oxides, were screened to identify catalytically active lattice oxygen structures for the partial oxidation of CH4 to formaldehyde and methanol. Fe/MoO3, Fe/V2O5, and particularly Fe/Nb2O5 were found to be highly effective. Structural analysis of the active Fe sites in the 11 supported Fe catalysts was performed using high-energy-resolution-fluorescence-detected Fe K-edge X-ray absorption near-edge structure spectroscopy, revealing that FeNbO4, FeMoO4, and FeVO4 species in Fe/Nb2O5, Fe/MoO3, and Fe/V2O5, respectively, are responsible for their partial-oxidation activities. In contrast, Fe2O3 species formed in Fe/Al2O3, Fe/SiO2, Fe/Ta2O5, and Fe/WO3 were found to be active for complete oxidation to CO2 than partial oxidation, as were the MgFe2O4, LaFeO3, and TiFe2O5 species formed in Fe/MgO, Fe/La2O3, and Fe/TiO2, respectively, and the interstitial solid solution of Fe3+ in ZrO2 generated in Fe/ZrO2. Furthermore, while the Fe2O3 species in Fe/WO4 are ineffective for partial oxidation, FeWO4 prepared by a hydrothermal method exhibits high selectivity for partial oxidation. Additionally, previous studies have shown that CuWO4 and CuMoO4 are active for partial CH4 oxidation. Accordingly, the ABO4 structure (where A is a 3d metal and B is a group 5 or 6 metal) is indicated as a viable design basis for the development of catalysts for partial CH4 oxidation.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


