Design, synthesis, and pharmacological evaluation of triazine-based PI3K/mTOR inhibitors for the potential treatment of non-small cell lung cancer
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Dysregulated activation of the PI3K/AKT/mTOR pathway is crucial in the development of cancer, and disrupting it could potentially lead to cancer suppression, making it a viable strategy for cancer treatment. Here, as a consecutive work of our team, we described the identification and optimization of PI3K/mTOR inhibitors based on triazine scaffold, which exhibited potent PI3K/mTOR inhibitor activity. The systematically structure-activity relationship (SAR) results demonstrated that compound 5nh displayed high efficacy against PI3Kα and mTOR, with the IC50 values of 0.45 nM and 2.9 nM, respectively. Importantly, compared to the lead compound PKI-587, 5nh demonstrated significant inhibitory activity against non-small-cell lung cancer (NSCLC) cell lines, particularly HCC-827, with a 43-fold increase (3.5 nM vs 150 nM). Additionally, the compound showed effective inhibition against the EGFR-resistant variant HCC-827(GR) cell line. Mechanism validation demonstrated that 5nh significantly interfered with the PI3K/AKT/mTOR signaling pathway in HCC-827 cells. Furthermore, the oral pharmacokinetic properties of 5nh had been observably improved, with AUC0-t and Cmax increasing by 13–16 times at a dose of 10 mg/kg in mice. Importantly, the in vivo efficacy study demonstrated that orally treatment of 5nh led to significant tumor growth suppression, with a TGI value of 84.4 %. Collectively, our systematically medicinal chemistry campaigns suggested that 5nh, a novel oral available triazine derivative, held promise as a candidate for therapy of NSCLC by targeting the PI3K/AKT/mTOR cascade.
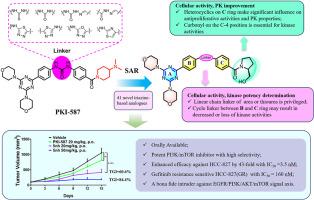

基于三嗪的PI3K/mTOR抑制剂治疗非小细胞肺癌的设计、合成和药理学评价
PI3K/AKT/mTOR通路的失调激活在癌症的发展中至关重要,破坏它可能会导致癌症抑制,使其成为癌症治疗的可行策略。在这里,作为我们团队的连续工作,我们描述了基于三嗪支架的PI3K/mTOR抑制剂的鉴定和优化,该支架表现出强大的PI3K/mTOR抑制剂活性。系统构效关系(SAR)结果表明,化合物5nh对PI3Kα和mTOR具有较强的抑制作用,IC50值分别为0.45 nM和2.9 nM。重要的是,与先导化合物PKI-587相比,5nh对非小细胞肺癌(NSCLC)细胞系,特别是HCC-827,具有显著的抑制活性,增加了43倍(3.5 nM比150 nM)。此外,该化合物对EGFR-resistant variant HCC-827(GR)细胞系有有效的抑制作用。机制验证表明5nh显著干扰HCC-827细胞PI3K/AKT/mTOR信号通路。此外,5nh的口服药代动力学性质明显改善,10 mg/kg剂量下AUC0-t和Cmax在小鼠体内增加13-16倍。重要的是,体内疗效研究表明,口服5nh可显著抑制肿瘤生长,TGI值为84.4%。总的来说,我们系统的药物化学活动表明,5nh,一种新型口服三嗪衍生物,通过靶向PI3K/AKT/mTOR级联,有望成为治疗非小细胞肺癌的候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


