Discovery of Novel Bicyclic and Tricyclic Cyclohepta[b]thiophene Derivatives as Multipotent AChE and BChE Inhibitors, In-Vivo and In-Vitro Assays, ADMET and Molecular Docking Simulation
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
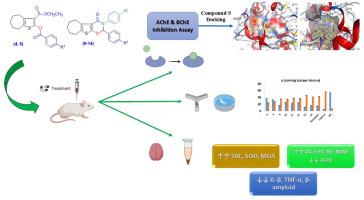
Alzheimer’s disease (AD) is primarily caused by oxidative stress, hyperphosphorylated τ-protein aggregation, and amyloid-β deposition. Changes in dopaminergic and serotoninergic neurotransmitter pathways are linked to certain symptoms of AD. Derivatives of bicyclic and tricyclic cyclohepta[b]thiophene were developed to identify new potential candidates as acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitors for the treatment of AD. All synthesized compounds exhibited AChE inhibition with IC50 values below 15 μM, while all compounds exhibited BChE inhibition with IC50 values below 25 μM. Compounds 9 and 12 exhibited AChE inhibitory activities with IC50 values of 0.51 μM and 0.55 μM, respectively. Compounds 5 and 9 demonstrated excellent inhibitory activity against BChE with IC50 values of 2.9 μM and 2.48 μM, respectively. Compounds 9, 13, and 14 were found to be the most active in terms of the decrease in the escape latency time, with values comparable to that of Donepezil. Compounds 10, 11, and 12 exhibited promising effects on learning and memory. Compounds 5, 10, 11, and 12 exhibited promising SAP values of 70.67%, 71.5%, 74.33% and 73.83%, respectively. Other biomarkers were evaluated in rat brains including TAC, MDA, SOD, BDNF, IL-β and TNF-α. Fundamental features of ADMET have been computed in-silico for synthesized compounds. Molecular docking was performed to confirm the binding of the novel compounds to the targets.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


