Corosolic acid and its derivatives targeting MCCC1 against insulin resistance and their hypoglycemic effect on type 2 diabetic mice
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Corosolic acid (CA), a natural triterpenoid, exhibits various biological activities and is often called as plant-derived insulin due to its significant hypoglycemic effects, making it especially beneficial for individuals with diabetes or high blood glucose levels. However, CA has notable in vitro toxicity, low water solubility, and poor pharmacokinetic properties. To address these limitations, a series of CA derivatives were synthesized, resulting in the identification of derivative H26, which demonstrates a significantly enhanced hypoglycemic effect, reduced toxicity, and improved pharmacokinetic characteristics compared to CA. To identify the target protein of CA and investigate its therapeutic potential, a chemical probe derived from natural products, called CA-biotin, was designed and synthesized. By employing an avidin-biotin affinity binding system, we distinguished the differential protein bands between CA-biotin and biotin. This quantitative proteomic analysis revealed, for the first time, that the biotin-containing enzyme methylcrotonoyl-CoA carboxylase 1 (MCCC1) directly binds to CA. The interaction between H26 and MCCC1 was examined in vitro. The research on the mechanisms by which CA and H26 address Type 2 diabetes mellitus (T2DM) focused on the insulin resistance signaling pathway, specifically targeting MCCC1. The results indicated that H26 shows significant promise as a potential hypoglycemic agent, while MCCC1 may serve as a valuable target for addressing insulin resistance. This presents a promising opportunity for developing new medications aimed at improving the health of patients with type 2 diabetes mellitus (T2DM) or hyperglycemia.
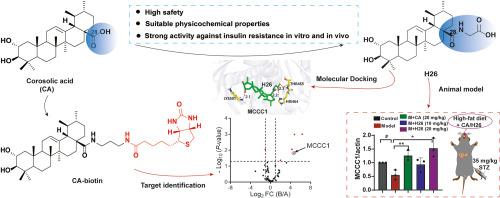

靶向MCCC1的科罗索酸及其衍生物对2型糖尿病小鼠胰岛素抵抗的影响及其降糖作用
Corosolic acid (CA)是一种天然的三萜,具有多种生物活性,由于其显著的降血糖作用,通常被称为植物源胰岛素,对糖尿病或高血糖患者特别有益。然而,CA具有明显的体外毒性、低水溶性和较差的药代动力学性质。为了解决这些限制,我们合成了一系列CA衍生物,并鉴定出衍生物H26,与CA相比,它具有显著增强的降糖作用,降低毒性,改善药代动力学特性。为了鉴定CA的靶蛋白并研究其治疗潜力,我们设计并合成了一种源自天然产物的化学探针CA生物素。通过亲和生物素结合系统,我们区分了ca生物素和生物素之间的差异蛋白带。该定量蛋白质组学分析首次揭示了含有生物素的酶甲基crotonoyl- coa羧化酶1 (MCCC1)直接与CA结合,并在体外检测了H26与MCCC1的相互作用。CA和H26治疗2型糖尿病(T2DM)的机制研究主要集中在胰岛素抵抗信号通路,特异性针对MCCC1。结果表明,H26作为一种潜在的降糖药具有重要的前景,而MCCC1可能作为解决胰岛素抵抗的有价值的靶点。这为开发旨在改善2型糖尿病(T2DM)或高血糖患者健康的新药物提供了一个有希望的机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


