Electrochemical performance of Al-Mg-Ga-Sn-In-Ce alloy as anodes for high-performance Al-AgO batteries
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
Addressing the challenges of passivation and self-corrosion in aluminum anodes is crucial for advancing aqueous Al-AgO battery performance. Herein, a multielement Al-based anode with suitable heat treatment is proposed. The influence of annealing on the microstructure, corrosion resistance, and discharge behavior of Al-1Mg-0.5Ga-0.5Sn-0.2In-0.02Ce (wt%) alloys in alkaline solutions is investigated via microstructure characterization and electrochemical measurements. The results indicate that appropriate annealing improves the homogeneity of grain structure and distribution of second phases as well as the formation of favorable textures. The microstructural evolution accordingly enhances the electrochemical performance of the anodes by facilitating uniform and rapid dissolution during discharge. The 3h-annealed alloy strikes an optimal balance between discharge activity and corrosion resistance at high current densities. It displays an average voltage and an anode efficiency of 1.654 V and 72.6 % at 900 mA cm-2, reaching a peak energy density of 3578.40 mWh g-1—an increase of 12 % compared to the as-rolled alloy. This enhancement is attributed to the moderate intergranular corrosion and the high-activity Cube{001}<100> orientation. The activation mechanism of the anodes is further elucidated. This work highlights that Al-Mg-Ga-Sn-In-Ce alloys are promising anode materials for underwater energy systems.
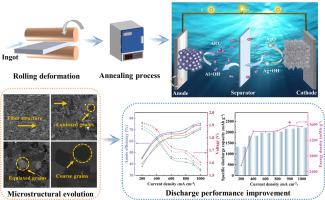
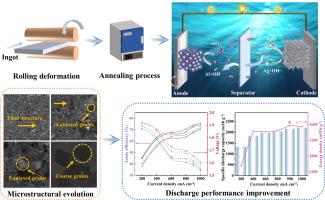
Al-Mg-Ga-Sn-In-Ce合金作为高性能Al-AgO电池阳极的电化学性能
解决铝阳极的钝化和自腐蚀问题对于提高Al-AgO水性电池的性能至关重要。为此,提出了一种经过适当热处理的多元素铝基阳极。通过组织表征和电化学测量,研究了退火对Al-1Mg-0.5Ga-0.5Sn-0.2In-0.02Ce (wt%)合金在碱性溶液中的组织、耐蚀性和放电行为的影响。结果表明,适当的退火可以改善晶粒组织和第二相分布的均匀性,有利于织构的形成。微观结构的演变通过促进放电过程中均匀快速的溶解而提高了阳极的电化学性能。在高电流密度下,3h退火合金在放电活性和耐腐蚀性之间达到了最佳平衡。在900 mA cm-2时,其平均电压和阳极效率分别为1.654 V和72.6%,峰值能量密度为3578.40 mWh g-1,比轧制态合金提高了12%。这种增强是由于适度的晶间腐蚀和高活性立方{001}<;100>;取向。进一步阐明了阳极的活化机理。这项工作强调了Al-Mg-Ga-Sn-In-Ce合金是一种很有前途的水下能源系统阳极材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


