Metabolomic interpretation of bacterial and fungal contribution to per- and polyfluoroalkyl substances interface migration in waterlogged paddy fields
IF 7.6
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Per- and polyfluoroalkyl substances (PFAS) are widely distributed in paddy soils, and their multi-phase partitioning in soil fractions was proved to be strongly interact with soil microbial community composition and functions. Despite this, soil bacterial and fungal metabolic molecular effects on PFAS water-soil interface migration in waterlogged paddy fields still remain unclear. This study integrated soil untargeted metabolomics with microbial amplicon sequencing to elucidate soil metabolic modulations of 15 PFAS interface release. Inhibition of bacterial and fungal metabolic activity both significantly altered PFAS cross-media translocation (p < 0.05). Gemmatimonadota, Desulfobacterota, Acidobacteriota, Actinobacteriota, and Bacteroidota were vital bacterial taxa affecting PFAS transport, while Basidiobolomycota and Chytridiomycota were vital fungal taxa. Fungi regulated PFAS migration more (R2 = 0.379–0.526) than bacteria (R2 = 0.021–0.030) due to the higher metabolic stability of stochastic-dominated fungi than deterministic-dominated bacteria. At the water-soil interface, the amino acid-like dissolved organic matter (Tyrosine and Tryptophan) contributed most (48.5–58.6 %) to the PFAS multiphase distribution. Untargeted metabolomics further clarified that fungal amino acid-like metabolites, including Phosphoenolpyruvate and Methionine, were key triggers stimulating Tyrosine and Tryptophan biosynthesis (p < 0.001), which were vital in modulating PFAS interface translocation (p < 0.001). These results provide novel insights into soil microbial metabolites’ participation in PFAS water-soil interface migration, benefiting PFAS pollution control and agricultural security risk assessment in waterlogged paddy ecosystems.
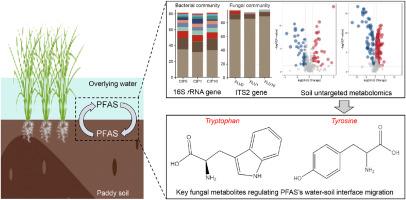

水田中细菌和真菌对单氟烷基和多氟烷基物质界面迁移的代谢组学解释
全氟烷基和多氟烷基物质(PFAS)广泛分布于水稻土中,它们在土壤组分中的多相分配与土壤微生物群落组成和功能有很强的相互作用。尽管如此,土壤细菌和真菌代谢分子对涝渍稻田中PFAS水土界面迁移的影响尚不清楚。本研究将土壤非靶向代谢组学与微生物扩增子测序相结合,阐明15种PFAS界面释放的土壤代谢调节。细菌和真菌代谢活性的抑制都显著改变了PFAS的跨媒介易位(p <;0.05)。双胞菌门、脱硫菌门、酸性菌门、放线菌门和拟杆菌门是影响PFAS运输的重要细菌类群,担子菌门和壶菌门是影响PFAS运输的重要真菌类群。真菌对PFAS迁移的调节作用(R2 = 0.379 ~ 0.526)大于细菌(R2 = 0.021 ~ 0.030),这是由于随机优势型真菌的代谢稳定性高于确定性优势型细菌。在水-土壤界面,类氨基酸溶解有机质(酪氨酸和色氨酸)对PFAS多相分布贡献最大(48.5 ~ 58.6%)。非靶向代谢组学进一步阐明真菌氨基酸样代谢物,包括磷酸烯醇丙酮酸和蛋氨酸,是刺激酪氨酸和色氨酸生物合成的关键触发物(p <;0.001),这对调节PFAS界面移位至关重要(p <;0.001)。这些结果为土壤微生物代谢物参与PFAS水-土界面迁移提供了新的认识,有助于涝渍稻田生态系统PFAS污染控制和农业安全风险评估。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


