Antigen presentation by tumor-associated macrophages drives T cells from a progenitor exhaustion state to terminal exhaustion
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Whereas terminally exhausted T (Tex_term) cells retain anti-tumor cytotoxic functions, the frequencies of stem-like progenitor-exhausted T (Tex_prog) cells better reflect immunotherapeutic responsivity. Here, we examined the intratumoral cellular interactions that govern the transition to terminal T cell exhaustion. We defined a metric reflecting the intratumoral progenitor exhaustion-to-terminal exhaustion ratio (PETER), which decreased with tumor progression in solid cancers. Single-cell analyses of Tex_prog cells and Tex_term cells in glioblastoma (GBM), a setting of severe T cell exhaustion, revealed disproportionate loss of Tex_prog cells over time. Exhaustion concentrated within tumor-specific T cell subsets, with cognate antigen exposure requisite for acquisition of the Tex_term phenotype. Tumor-associated macrophages (TAMs)—not tumor cells—were the primary source of antigenic exposure governing the Tex_prog to Tex_term transition. TAM depletion increased frequencies of Tex_prog cells in multiple tumor models, increased PETER, and promoted responsiveness to αPD1 immunotherapy. Thus, targeting TAM-T cell interactions may further license checkpoint blockade responses.
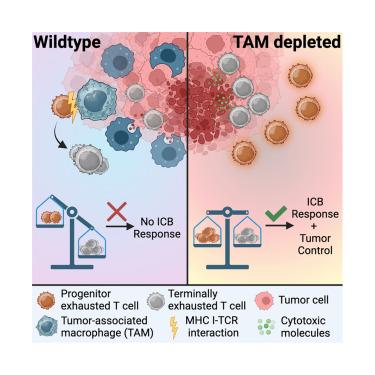
肿瘤相关巨噬细胞的抗原呈递驱动T细胞从祖细胞衰竭状态到终末衰竭状态
虽然终端耗竭T (Tex_term)细胞保留抗肿瘤细胞毒性功能,但干细胞样祖耗竭T (Tex_prog)细胞的频率更好地反映了免疫治疗反应性。在这里,我们研究了肿瘤内的细胞相互作用,这些相互作用控制着向终端T细胞衰竭的过渡。我们定义了一个反映肿瘤内祖细胞耗竭与终末耗竭比(PETER)的指标,该指标在实体癌中随着肿瘤进展而降低。对胶质母细胞瘤(GBM)中Tex_prog细胞和Tex_term细胞的单细胞分析显示,随着时间的推移,Tex_prog细胞的损失不成比例。衰竭集中在肿瘤特异性T细胞亚群中,同源抗原暴露是获得Tex_term表型所必需的。肿瘤相关巨噬细胞(tam) -而非肿瘤细胞-是控制Tex_prog到Tex_term转变的抗原暴露的主要来源。TAM缺失增加了多种肿瘤模型中Tex_prog细胞的频率,增加了PETER,并促进了对αPD1免疫治疗的反应性。因此,靶向TAM-T细胞相互作用可能进一步许可检查点阻断反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


