Risk of infections during treatment with oral Janus kinase inhibitors in randomized placebo-controlled trials: A systematic review and meta-analysis
IF 5.2
引用次数: 0
Abstract
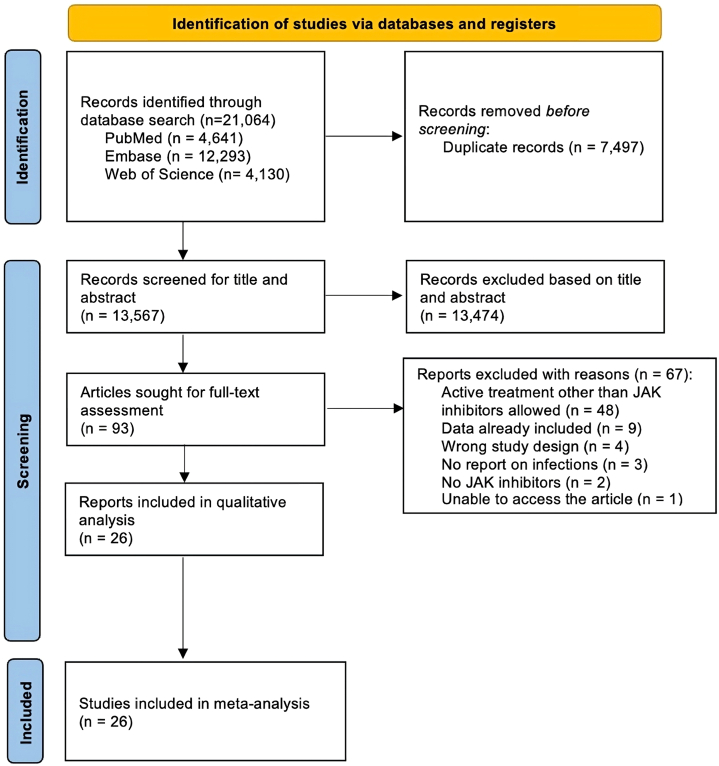
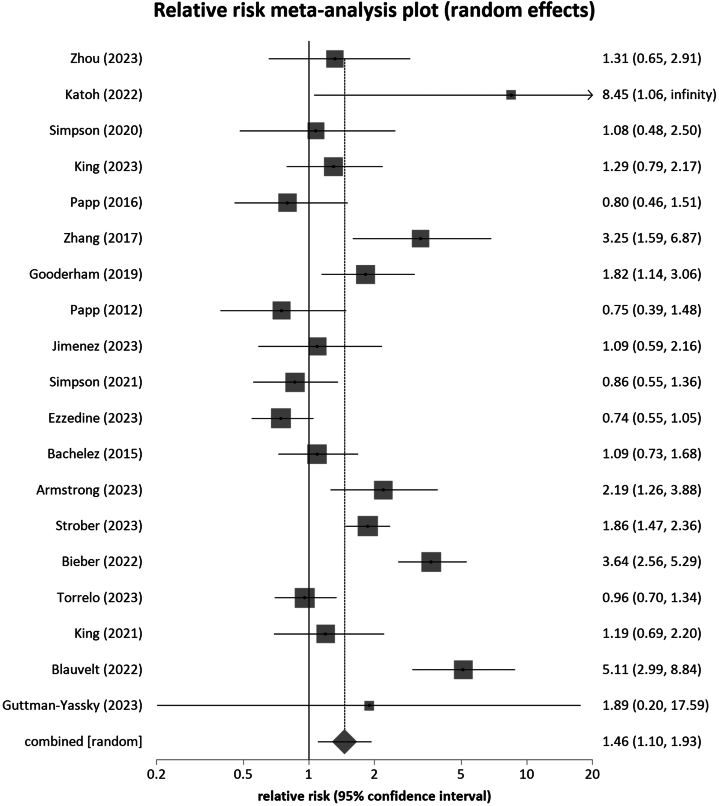
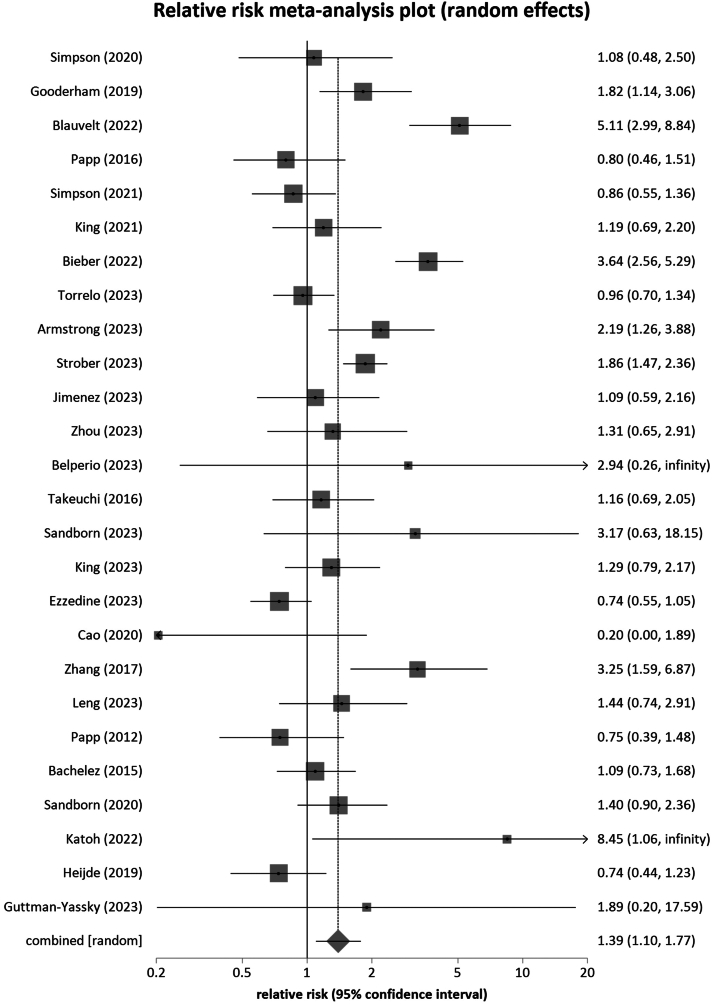
Janus kinase (JAK) inhibitors block pathways involved in inflammation and immune response, making JAK inhibitors useful in the treatment of various diseases. While the efficacy of these drugs has been proven in several studies, their safety profile needs to be further investigated. In this systematic review and meta-analysis, we examined the risk of infections during treatment with oral JAK inhibitors with no concomitant treatment compared to placebo in phase 2 and 3 randomized, placebo-controlled trials. The medical databases PubMed, Web of Science, and EMBASE were searched from inception through February 2024, yielding 13,567 nonduplicate articles, of which 69 were included in the final quantitative analysis. Overall, we found that treatment with oral JAK inhibitors was associated with an increased risk of infections compared to placebo across all indications (relative risk: 1.39 [95% CI: 1.096-1.76, P = .0067]) and in dermatologic indications (relative risk: 1.46 [95% CI, 1.10-1.93, P = .0097]). Remarkably, an increased risk of herpes zoster infections was found in dermatologic indications but not in nondermatologic indications. In conclusion, we identified a significantly increased risk of developing infections during treatment with oral JAK inhibitors compared to placebo across indications. In sub-analyses, we additionally found an increased risk of herpes zoster in dermatologic indications.
随机安慰剂对照试验中口服Janus激酶抑制剂治疗期间感染的风险:系统回顾和荟萃分析。
Janus激酶(JAK)抑制剂阻断炎症和免疫反应的通路,使JAK抑制剂在治疗各种疾病中有用。虽然这些药物的有效性已在几项研究中得到证实,但其安全性需要进一步调查。在这项系统综述和荟萃分析中,我们在2期和3期随机、安慰剂对照试验中比较了口服JAK抑制剂治疗期间未合并治疗与安慰剂治疗期间的感染风险。从开始到2024年2月,检索了医学数据库PubMed、Web of Science和EMBASE,得到13567篇非重复文章,其中69篇被纳入最终的定量分析。总的来说,我们发现口服JAK抑制剂治疗与安慰剂相比,在所有适应症(相对危险度:1.39 [95% CI: 1.096-1.76, P = 0.0067])和皮肤病适应症(相对危险度:1.46 [95% CI, 1.10-1.93, P = 0.0097])中感染风险增加。值得注意的是,带状疱疹感染的风险增加是在皮肤指征中发现的,而不是在非皮肤指征中。总之,我们发现口服JAK抑制剂治疗期间发生感染的风险显著增加,与安慰剂的适应症相比。在亚分析中,我们还发现皮肤指征中带状疱疹的风险增加。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


