Tuft cells transdifferentiate to neural-like progenitor cells in the progression of pancreatic cancer
IF 10.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Pancreatic ductal adenocarcinoma (PDA) is partly initiated through the transdifferentiation of acinar cells to metaplasia, which progresses to neoplasia and cancer. Tuft cells (TCs) are chemosensory cells not found in the normal pancreas but arise in cancer precursor lesions and diminish during progression to carcinoma. These metaplastic TCs (mTCs) suppress tumor progression through communication with the tumor microenvironment, but their fate during progression is unknown. To determine the fate of mTCs during PDA progression, we created a dual recombinase lineage trace model, wherein a pancreas-specific FlpO was used to induce tumorigenesis, while a tuft-cell specific Pou2f3CreERT/+ driver was used to induce expression of a tdTomato reporter. We found that mTCs in carcinoma transdifferentiate into neural-like progenitor cells (NRPs), a cell type associated with poor survival in patients. Using conditional knockout and overexpression systems, we found that Myc activity in mTCs is necessary and sufficient to induce this tuft-to-neuroendocrine transition (TNT).
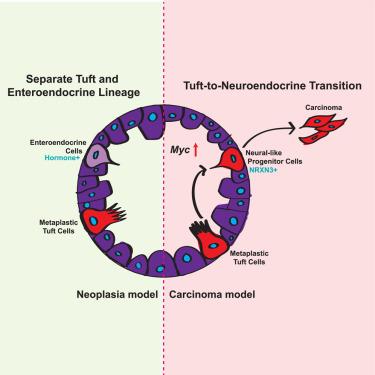
在胰腺癌的发展过程中,簇状细胞向神经样祖细胞转化
胰腺导管腺癌(PDA)部分是通过腺泡细胞转分化为化生而开始的,化生发展为肿瘤和癌症。簇状细胞(TCs)是一种化学感觉细胞,在正常胰腺中没有发现,但在癌前病变中出现,并在癌进展过程中减少。这些化生tc (mTCs)通过与肿瘤微环境的交流抑制肿瘤进展,但它们在进展过程中的命运尚不清楚。为了确定PDA进展过程中mTCs的命运,我们创建了一个双重组酶谱系追踪模型,其中胰腺特异性FlpO用于诱导肿瘤发生,而簇细胞特异性Pou2f3CreERT/+驱动程序用于诱导tdTomato报告基因的表达。我们发现癌中的mTCs转分化为神经样祖细胞(NRPs),这是一种与患者生存率低相关的细胞类型。通过条件敲除和过表达系统,我们发现mTCs中的Myc活性是诱导这种簇向神经内分泌转化(TNT)的必要和充分条件。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Developmental cell
生物-发育生物学
CiteScore
18.90
自引率
1.70%
发文量
203
审稿时长
3-6 weeks
期刊介绍:
Developmental Cell, established in 2001, is a comprehensive journal that explores a wide range of topics in cell and developmental biology. Our publication encompasses work across various disciplines within biology, with a particular emphasis on investigating the intersections between cell biology, developmental biology, and other related fields. Our primary objective is to present research conducted through a cell biological perspective, addressing the essential mechanisms governing cell function, cellular interactions, and responses to the environment. Moreover, we focus on understanding the collective behavior of cells, culminating in the formation of tissues, organs, and whole organisms, while also investigating the consequences of any malfunctions in these intricate processes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


