Outcomes of Patients With Treated Secondary Acute Myeloid Leukemia: A High-Risk Subtype That Warrants an Independent Prognostic Designation
IF 10.1
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
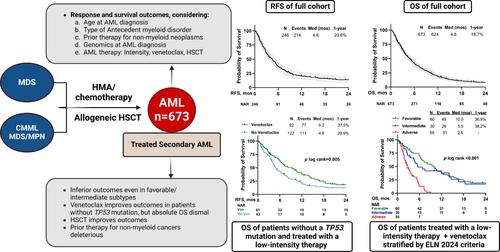
Patients who develop acute myeloid leukemia (AML) after having received treatment for myelodysplastic syndrome (MDS) or related conditions have particularly poor outcomes. This study analyzed adult patients with newly diagnosed AML who previously had MDS, chronic myelomonocytic leukemia (CMML), or MDS/myeloproliferative neoplasm (MPN) overlap syndrome, and who had received hypomethylating agents, chemotherapy, and/or allogeneic stem cell transplantation (HSCT) for these antecedent disorders. From January 2012 to August 2023, we included 673 patients with a median age of 70 years (range, 19–94); 536 (80%) had transformed from MDS, and the remainder from CMML or MDS-MPN. Additionally, 149 patients (22%) had prior therapy for nonmyeloid malignancies. Among 497 evaluable patients, 289 (58%) had adverse-risk (AR) cytogenetics, 34% had TP53 mutation/s, and 71% were classified as AR by the ELN 2017 criteria. Most patients (67%) received low-intensity therapy (LIT) for AML, and 27% were treated with venetoclax. The overall response rate was 37%, and venetoclax improved the odds of response (OR = 2.5, 95% CI 1.6–3.7) in LIT–treated patients. At a median follow-up of 43 months, the median relapse-free survival (RFS) and overall survival (OS) were 4.6 and 4.8 months, respectively. Multivariate analysis showed that prior therapy for nonmyeloid disorders (HR = 1.30), ≥ 2 lines of therapy for antecedent myeloid disorders (HR = 1.23), and ELN AR risk (HR = 1.47) increased the hazards of death, while HSCT (HR = 0.50) was beneficial and validated on gradient-boosted regression. TS-AML is associated with poor outcomes irrespective of AML genomics and treatment, highlighting the need for its inclusion as an independent AR category for accurate prognostication and clinical trial reporting.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
15.70
自引率
3.90%
发文量
363
审稿时长
3-6 weeks
期刊介绍:
The American Journal of Hematology offers extensive coverage of experimental and clinical aspects of blood diseases in humans and animal models. The journal publishes original contributions in both non-malignant and malignant hematological diseases, encompassing clinical and basic studies in areas such as hemostasis, thrombosis, immunology, blood banking, and stem cell biology. Clinical translational reports highlighting innovative therapeutic approaches for the diagnosis and treatment of hematological diseases are actively encouraged.The American Journal of Hematology features regular original laboratory and clinical research articles, brief research reports, critical reviews, images in hematology, as well as letters and correspondence.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


