Total Synthesis of Exiguolide Stereoisomers: Impact of Stereochemical Permutation on Reactivity, Conformation, and Biological Activity
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
(−)-Exiguolide is a marine macrolide natural product with potent anticancer activity. In this study, the total synthesis of exiguolide stereoisomers, (9R)-exiguolide, (9R,13S)-exiguolide, and (9R,13S,19R)-exiguolide, was achieved by capitalizing on our macrocyclization/transannular pyran cyclization strategy. The impact of the stereochemical permutation on the reactivity of advanced intermediates, the conformation of the macrocyclic skeleton, and the antiproliferative activity against human cancer cells were investigated in detail. The total synthesis of (9R,13S)-exiguolide and (9R,13S,19R)-exiguolide was completed in much the same way as that of the parent natural product using stereoisomeric building blocks. Nevertheless, the reactivity of the (9R,13S)- and (9R,13S,19R)-series of intermediates in macrocyclic ring-closing metathesis and transannular pyran-forming reactions was significantly different from that of naturally configured counterparts. The conformation of exiguolide stereoisomers, deduced by means of NMR spectroscopic analysis and DFT calculations, was clearly different from that of the parent natural product. Evaluation of the antiproliferative activity of exiguolide and its stereoisomers suggested the importance of the stereochemistry of the macrocyclic skeleton.
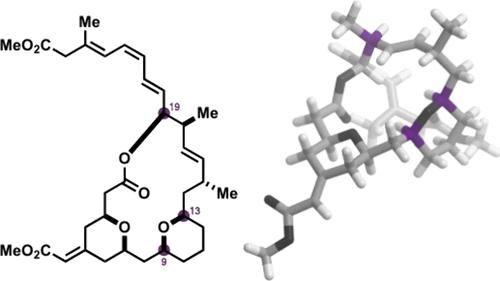
烯瓜内酯立体异构体的全合成:立体化学排列对反应性、构象和生物活性的影响
(−)-Exiguolide是一种具有强抗癌活性的海洋大环内酯天然产物。在本研究中,利用我们的大环化/跨环吡喃环化策略,完成了外郭内酯立体异构体(9R)-外郭内酯、(9R,13S)-外郭内酯和(9R,13S,19R)-外郭内酯的合成。详细研究了立体化学排列对高级中间体反应活性、大环骨架构象以及对人类癌细胞的抗增殖活性的影响。(9R,13S)-逸出iguolide和(9R,13S,19R)-逸出iguolide的全合成方法与母体天然产物的合成方法基本相同。然而,(9R,13S)-和(9R,13S,19R)-系列中间体在大环闭合环复合和跨环吡喃形成反应中的反应活性与自然构型的中间体有显著差异。通过核磁共振波谱分析和离散傅立叶变换(DFT)计算,得出了外果油内酯立体异构体的构象与母体天然产物的构象明显不同。槲皮内酯及其立体异构体的抗增殖活性的评价表明了立体化学对大环骨架的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


