Sulfide and Hydrogen Bond Networks in the Electric Double Layer: Key Factors for Titanium Passivation Film Stability
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Hydrogen sulfide (H2S), carbonyl sulfide (COS), and dimethyl sulfide (DMS) are the primary sulfur compounds found in seawater, which cause pitting corrosion on the oxide passivation film of titanium, known as “the marine metals”. In this study, density functional theory (DFT) was used to analyze the adsorption and surface electronic properties of these three small molecules on the anatase TiO2(101) surface. The analysis was conducted through adsorption energy, work function, Mulliken charge population, and density of states (DOS). The hydrogen bond network structure of the electric double layer (EDL) was studied for these small-molecule systems using ab initio molecular dynamics (AIMD). The optimal adsorption configurations for H2S, COS, and DMS on the anatase TiO2(101) surface are 2Ob-vertical, O-down-vertical, and Ob-parallel, with adsorption energies of −1.32, −0.67, and −1.86 eV, respectively. The surface charge transfer was also investigated. Through comparative AIMD simulations of three different aqueous solutions on the TiO2(101) surface, we observed that COS exerts a more pronounced influence on the electrical double layer within 3.00 Å of the TiO2(101) surface. Specifically, the hydrogen atoms of water tend to aggregate toward the Ob atoms, forming hydrogen bonds, which significantly impacts the corrosion resistance of the TiO2 surface.
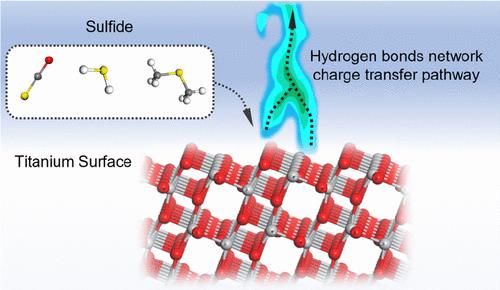
双电层中硫化物和氢键网络:影响钛钝化膜稳定性的关键因素
硫化氢(H2S)、羰基硫化物(COS)和二甲基硫化物(DMS)是海水中的主要含硫化合物,它们对钛的氧化钝化膜产生点蚀作用,被称为“海洋金属”。本研究采用密度泛函理论(DFT)分析了这三种小分子在锐钛矿TiO2(101)表面的吸附和表面电子性质。通过吸附能、功函数、Mulliken电荷居数和态密度(DOS)进行分析。利用从头算分子动力学(AIMD)对这些小分子体系的双电层(EDL)氢键网络结构进行了研究。H2S、COS和DMS在锐钛矿TiO2(101)表面的最佳吸附构型为垂直2ob、垂直o向下和平行ob,吸附能分别为- 1.32、- 0.67和- 1.86 eV。对表面电荷转移也进行了研究。通过对TiO2(101)表面三种不同水溶液的AIMD模拟对比,我们发现COS对TiO2(101)表面3.00 Å范围内双电层的影响更为显著。具体来说,水的氢原子倾向于向Ob原子聚集,形成氢键,这显著影响了TiO2表面的耐腐蚀性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


