Modulating the Coordination Environment of Cu-Embedded MoX2 (X = S, Se, and Te) Monolayers for Electrocatalytic Reduction of CO2 to CH4: Unveiling the Origin of Catalytic Activity
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The electrochemical CO2 reduction reaction (CO2RR) is a promising approach to alleviating global warming and emerging energy crises. Yet, the CO2RR efficiency is impeded by the need for electrocatalysts with good selectivity and efficiency. Recently, single-atom catalysts (SACs) have attracted much attention in electrocatalysis and are more efficient than traditional metal-based catalysts. In this study, we modeled a Cu single atom embedded on MoX2 (X = Se and Te) monolayer with a single chalcogen (X) vacancy as SAC. Employing the dispersion-corrected density functional theory (DFT-D3) method, the electrocatalytic CO2RR activity of the Cu-MoX2 SACs is systematically investigated through significant descriptors, such as the Gibbs free energy change, charge density difference, and COHP analysis. The stability of SACs, CO2 adsorption configurations, and all possible reaction pathways for the formation of C1 products (HCOOH, CO, CH3OH, and CH4) were examined. All of the Cu-MoX2 SACs are stable and show high catalytic selectivity for the CO2RR by significantly suppressing the hydrogen evolution reaction (HER). We found that the catalytic activity is mainly due to the level of antibonding states filling between the Cu atom and *OCHOH intermediate. Among the C1 products, CH4 is selectively produced in all three SACs. Notably, there is a decrease in the limiting potential (UL) when X changes from S to Te in Cu-MoX2. Among these three SACs, Cu-MoTe2 SAC is the most promising catalyst for reducing CO2 to CH4, with as low as UL of −0.34 V vs RHE. Our results demonstrate that the local coordination environment in SACs has a significant impact on the catalytic activity of CO2RR.
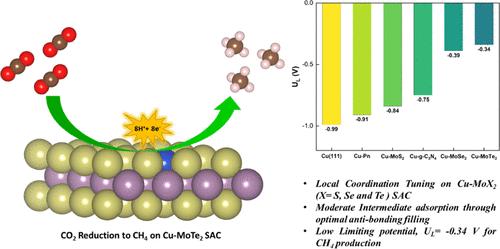
调节cu包埋MoX2 (X = S, Se, Te)单层电催化还原CO2制CH4的配位环境:揭示催化活性的来源
电化学CO2还原反应(CO2RR)是缓解全球变暖和新兴能源危机的一种有前景的方法。然而,由于需要具有良好选择性和效率的电催化剂,阻碍了CO2RR效率的提高。近年来,单原子催化剂因其比传统金属基催化剂更高效而在电催化领域受到广泛关注。在这项研究中,我们模拟了一个Cu单原子嵌套在MoX2 (X = Se和Te)单层上,并且有一个单个的chogen (X)空位作为SAC。采用色散校正密度泛函理论(DFT-D3)方法,通过Gibbs自由能变化、电荷密度差和COHP分析等重要描述符,系统地研究了Cu-MoX2 SACs的电催化CO2RR活性。考察了SACs的稳定性、CO2吸附构型以及生成C1产物(HCOOH、CO、CH3OH和CH4)的所有可能反应途径。Cu-MoX2 SACs稳定且对CO2RR具有较高的催化选择性,可显著抑制析氢反应(HER)。我们发现催化活性主要取决于Cu原子和*OCHOH中间体之间的反键态填充水平。在C1产物中,CH4选择性地在三个sac中产生。值得注意的是,当X从S变为Te时,Cu-MoX2的极限电位(UL)降低。在这三种SAC中,Cu-MoTe2 SAC是最有希望将CO2还原为CH4的催化剂,其UL低至−0.34 V vs RHE。我们的研究结果表明,SACs中的局部配位环境对CO2RR的催化活性有显著影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


