Thermodynamic and Process Analyses of Extractive Distillation for Separating Refrigerant R-410A Using Imidazolium-Cyano-Based Ionic Liquids
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
R-410A is a near-azeotropic refrigerant mixture of difluoromethane (HFC-32) and pentafluoroethane (HFC-125) that is scheduled to be phased out internationally due to a high Global Warming Potential (GWP). While conventional distillation cannot be used, extractive distillation using ionic liquids (ILs) has been proposed to separate and recycle HFC-32 (lower GWP) for future refrigerant blends and to repurpose HFC-125 (high GWP). Here, we present both a thermodynamic and process design analyses of various ILs using an equation of state method. From experimental binary vapor–liquid equilibrium data and equation of state modeling, 1-ethyl-3-methylimidazolium tricyanomethanide [C2C1im][tcm] and 1-ethyl-3-methylimidazolium dicyanamide [C2C1im][dca] were identified from 10 ILs as viable entrainers. Selectivity increases with temperature and decreases with pressure, while the solvent-to-feed (S/F) ratio did not have much effect. These effects were more pronounced for [C2C1im][tcm] than for [C2C1im][dca]. A sensitivity analysis reveals that the optimum operational parameters were solvent-to-feed ratio (S/F), reflux ratio (RR), and total number of stages (Nt) with values of 5, 3, and 20, respectively, for the case of [C2C1im][tcm] and 6, 3, and 20 for [C2C1im][dca]. High-purity products HFC-32 and HFC-125 (>99.5 wt %) were obtained with IL [C2C1im][tcm], providing the lowest energy consumption, compared to [C2C1im][dca] and other ILs.
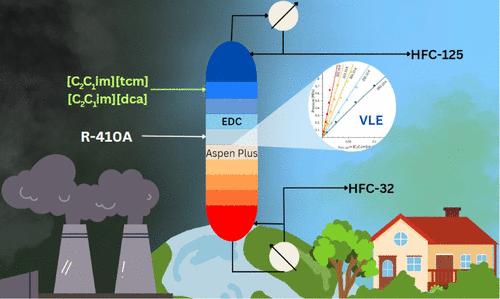
咪唑-氰基离子液体萃取精馏分离R-410A制冷剂的热力学及过程分析
R-410A是一种由二氟甲烷(HFC-32)和五氟乙烷(HFC-125)组成的近共沸制冷剂混合物,由于其全球变暖潜势(GWP)较高,计划在国际上逐步淘汰。虽然不能使用传统蒸馏,但已经提出使用离子液体(ILs)的萃取蒸馏来分离和回收HFC-32(低GWP)用于未来的制冷剂混合物,并重新利用HFC-125(高GWP)。在这里,我们提出了热力学和工艺设计的分析,利用状态方程的方法。根据实验二元气液平衡数据和状态方程模型,从10个il中确定了1-乙基-3-甲基咪唑三氰甲烷[C2C1im][tcm]和1-乙基-3-甲基咪唑双氰酰胺[C2C1im][dca]为可行的夹带剂。选择性随温度的升高而升高,随压力的增大而降低,而溶剂与进料比(S/F)的影响不大。与[C2C1im][dca]相比,[C2C1im][tcm]的效果更为明显。灵敏度分析表明,[C2C1im][tcm]的最佳操作参数为溶剂进料比(S/F)、回流比(RR)和总级数(Nt),分别为5、3和20,[C2C1im][dca]的最佳操作参数为6、3和20。与[C2C1im][dca]和其他IL相比,用IL [C2C1im][tcm]可获得高纯度产品HFC-32和HFC-125 (>99.5 wt %),能耗最低。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


