Generating a Peptide Library Using the Repeats of Amino Acid Scaffolds Created by Sliding the Framework of a 7-mer Human Chemerin Segment and Discovery of Potent Antibacterial and Antimycobacterial Peptides
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
The quest for new approaches for generating novel bioactive designer proteins/peptides has continued with their success in various biomedical applications. Previously, we designed a 14-mer α-helical peptide with antimicrobial and antimycobacterial activities by employing a tandem repeat of the 7-mer, “KVLGRLV” human chemerin segment. Herein, we devised a new method of “sliding framework” with this segment to create amino acid scaffolds of varying sizes and sequences and explored the design of a peptide library with antibacterial and antimycobacterial activities. By utilizing 2 to 7 repeats of these 2 to 6-residue scaffolds, we designed and synthesized 30 peptides of 10–16 residue lengths. Thus, we identified novel AMPs with α-helical, β-sheet, and random coil structures, membrane-destabilizing, and intracellular modes of action, and 9 of them showed therapeutic indices between 100 and 750. Three and two of these nine peptides showed in vivo antibacterial and antitubercular efficacies against Escherichia coli ATCC 25922 and Mycobacterium bovis BCG infections, respectively, in a mouse model.
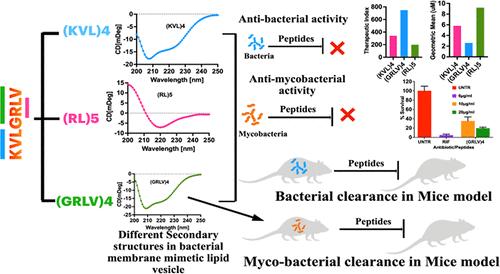
利用滑动7聚人趋化素片段框架产生的氨基酸支架的重复序列生成肽库和发现有效的抗菌和抗细菌肽
对产生新型生物活性设计蛋白/肽的新方法的探索,在各种生物医学应用中取得了成功。在此之前,我们设计了一个具有抗菌和抗细菌活性的14聚α-螺旋肽,通过串联重复7聚人趋化素片段“KVLGRLV”。在此,我们设计了一种新的“滑动框架”方法,利用该片段创建不同大小和序列的氨基酸支架,并探索了具有抗菌和抗细菌活性的肽库的设计。利用2 ~ 6个残基的2 ~ 7次重复,我们设计并合成了30个10 ~ 16个残基长度的肽段。因此,我们发现了具有α-螺旋、β-片状和随机线圈结构、膜不稳定和细胞内作用模式的新型amp,其中9种amp的治疗指数在100 - 750之间。在小鼠模型中,这9种肽中的3种和2种分别显示出对大肠杆菌ATCC 25922和牛卡介苗分枝杆菌感染的体内抗菌和抗结核作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


