Constructing Bridge Hydroxyl Groups on the Ru/MOx/HZSM-5 (M = W, Mo) Catalysts to Promote the Hydrolysis Oxidation of Multicomponent VOCs
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
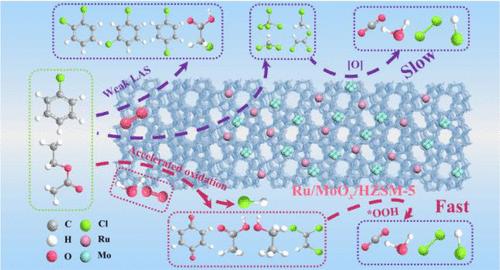
Chlorinated and oxygenated volatile organic compounds (CVOCs and OVOCs) pose a significant threat to human health. Catalytic oxidation effectively removes these pollutants, but catalyst deactivation is a challenge. Our study focused on the hydrolysis oxidation of chlorobenzene (CB) and ethyl acetate (EA) over Ru/MOx/HZSM-5 (M = W, Mo). It was found that doping MoOx to the catalyst increased the structural hydroxyl amount and balanced surface acidity, thus significantly improving the catalytic stability, with Ru/MoOx/HZSM-5 exhibiting a better activity for CB and EA oxidation (T90% = 438 and 276 °C at space velocity = 20,000 mL g–1 h–1, respectively). Water vapor introduction considerably promoted hydrolysis oxidation and protected the active sites from being poisoned by cumulative chlorine. The synergistic interaction of the Mo–O(H)–Al structure in Ru/MoOx/HZSM-5 with the Si–OH–Al structure promotes the activation of H2O to form bridging hydroxyl groups, which provide a proton-rich environment for hydrolysis oxidation. It was also found that dissociated H2O reacted with adsorbed oxygen species to form highly active *OOH, accelerating the deep oxidation of intermediates. We believe that the present study can provide a unique strategy for the effective elimination of multicomponent VOCs under complex conditions.
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


