Chlorpyrifos Influences Tadpole Development by Disrupting Thyroid Hormone Signaling Pathways
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
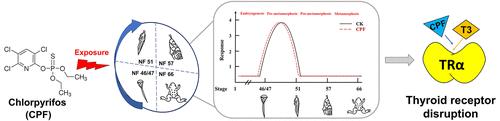
Chlorpyrifos (CPF) is a widely used organophosphate insecticide with serious toxicological effects on aquatic animals. Although extensively studied for neurotoxicity and endocrine disruption, its stage-specific effects on amphibian metamorphosis and receptor-level interactions remain unclear. This study investigated the effects of CPF on Xenopus laevis metamorphosis at environmentally relevant concentrations (1.8 and 18 μg/L) across key developmental stages, with end points including premetamorphic progression, thyroid hormone (TH)-responsive gene expression, and levels of triiodothyronine (T3) and thyroxine (T4). Additionally, molecular docking, surface plasmon resonance (SPR), and luciferase reporter gene assays were employed to elucidate CPF’s interaction with the thyroid hormone receptor alpha (TRα). CPF accelerated premetamorphic development and upregulated TH-responsive genes but delayed later-stage metamorphosis. After 21 days of exposure to 18 μg/L CPF, T3 and T4 levels were reduced by 28% and 39.4%, respectively, compared to controls. Cotreatment with T3 and CPF slowed tadpole development, indicating that CPF affects thyroid signaling in a stage-dependent manner. CPF competed with T3 for TRα binding and stimulated TRα-mediated luciferase activity when administered alone, but this activity decreased when CPF was coexposed to T3. These findings suggest that CPF functions as a partial agonist of TRα, disrupting thyroid signaling and adversely affecting amphibian development.
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


