Probing enantioinduction in confined chiral spaces through asymmetric oxime reductions
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Enzyme-like enantiopure supramolecular hosts leverage non-covalent and electrostatic interactions to engage substrates in a chiral environment without direct coordination. Elucidating the mechanistic underpinnings of enantioinduction in these systems is critical to the success of this nascent field. We report herein an enantiopure Ga4L612− host-catalyzed asymmetric reduction of aromatic, heteroaromatic, and aliphatic oximes to hydroxylamines, without N–O bond cleavage, using pyridine borane as a reductant cofactor. The reaction scope and mechanistic study, in combination with data science analysis, showcase that guest recognition and enantioinduction are highly sensitive to both steric and electronic effects. Optimization of interactions between the host, oxime, and reductant within the host cavity enabled highly enantioselective reactivity (>99% ee) for even previously unreported pyridine oximes. The emergent principles outlined herein lay the foundation for future applications of these promising catalytic scaffolds toward challenging synthetic targets.
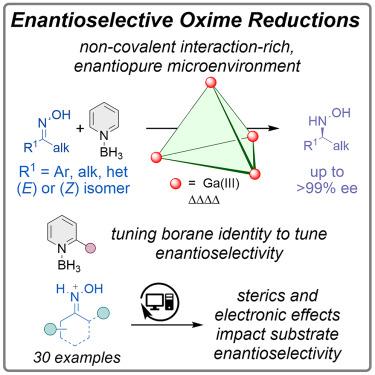

通过不对称肟还原在受限手性空间中探测对映体诱导
类酶对映纯超分子宿主利用非共价和静电相互作用在手性环境中参与底物而无需直接配位。阐明这些系统中对映体诱导的机制基础对这一新兴领域的成功至关重要。本文报道了一种对映纯Ga4L612−宿主催化的芳香、杂芳香和脂肪肟不对称还原成羟胺,不需要N-O键断裂,使用吡啶硼烷作为还原剂辅因子。反应范围和机理研究结合数据科学分析表明,客体识别和对映体诱导对空间和电子效应都高度敏感。优化宿主、肟和还原剂在宿主腔内的相互作用,即使是以前未报道的吡啶肟也具有高度的对映选择性反应活性(>99% ee)。本文概述的新兴原理为这些有前途的催化支架在具有挑战性的合成目标方面的未来应用奠定了基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


