Elementary Steps, Site Requirements, and Support Effects in Methylcyclohexane Dehydrogenation Reactions on Dispersed Pd Nanoparticles
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
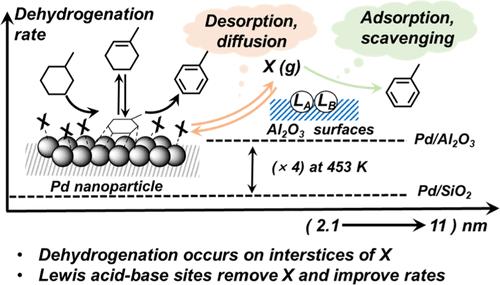
Hydrogenation-dehydrogenation cycles enable the efficient storage, transport, and release of hydrogen via chemical means. Practical kinetic, thermodynamic, and H-density considerations make cyclic hydrocarbons the preferred organic hydrogen carriers. This study addresses the mechanism of methylcyclohexane (MCH) dehydrogenation to toluene (TOL), through methylcyclohexene (MCHE) intermediates on Pd nanoparticles (2–11 nm diameter) dispersed on Al2O3, SiO2, MgO, and CeO2. Turnovers occur on Pd surfaces densely covered with MCH-derived intermediates differing in isomeric structure and reactivity via sequential C–H activation elementary events, irrespective of nanoparticle size or support. The kinetically relevant step shifts from the second to the first H-abstraction step in MCH as temperature increases (from 453 to 553 K). The reactivity of Pd nanoparticle surfaces is insensitive to their size but supports with more competent Lewis acid–base (LAB) pairs lead to higher rates and stronger rate enhancements (relative to SiO2) with decreasing temperatures, which reflect the lower coverages of less reactive intermediates when supports can scavenge desorbable species. These dense adlayers retain interstices within which dehydrogenation turnovers occur, but no longer expose the most distinctive low-coordination atoms prevalent on small nanoparticles, leading to the observed structure insensitivity of turnover rates. The prevalence of such adlayers leads to surfaces without the saturation hydrogen coverages expected for Pd surfaces devoid of such organic species. These mechanistic insights are consistent with (i) the elimination of support effect by titration of LAB pairs; (ii) initial rate transients that are inhibited by competent supports; (iii) the relative reactivity of metal-free supports for dehydrogenation of MCHE and methylcyclohexadienes (but not MCH); and (iv) measured kinetic effects of MCH, MCHE, and H2 on turnover rates. The support effects provide strategies for maximizing the exposure of bare atom ensembles during dehydrogenation reactions. Its conceptual impact and practical significance are not restricted to the subject reaction in this study.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


