The nature of interfacial catalysis over Pt/NiAl2O4: Effects of metal identity and CO tolerance in hydrogen production from methanol reforming reaction
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
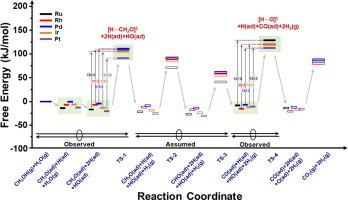
In this study, we investigate the effect of interfacial structure on the methanol steam reforming (MSR) reaction by systematically varying the transition metals (Pt, Ru, Rh, Pd, and Ir) over a NiAl2O4 support. At similar methanol conversions at ∼ 5 %, H2 formation exhibits superior reaction performance at ∼ 0.14 molH2·molmetal-1·s−1 over Pt/NiAl2O4 than any other counter parts, which was attributed to the enhanced ability in tolerance of CO from and catalytic surface and the greater ability in CH3O– activation with the activation energies of MSR, MD, and WGS at 70 kJ/mol, 79 kJ/mol, and 97 kJ/mol, respectively, which is obviously smaller than the other transition metal catalysts. More importantly, the higher reduction degree of Pt and more stabilized transition state along methanol activation might be additional reasons for this superior reaction performance on H2 formation.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


