High antimony resistance strain Enterobacter sp. Z1 mediates biomineralization of antimony trioxide
IF 10.3
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
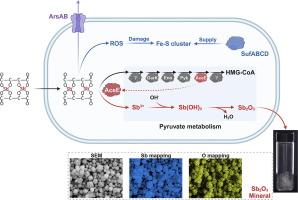
The increasing antimony (Sb) contamination prevalence poses a concern owing to its toxicity and potential carcinogenic properties. However, mechanisms underlying the microbial conversion of soluble Sb into insoluble Sb minerals remain unclear. In the present study, Enterobacter sp. Z1 strain demonstrated remarkable resistance to antimony potassium tartrate [Sb(III)] (>250 mM) in R2A medium. Furthermore, Enterobacter sp. Z1 produced antimony trioxide (Sb2O3) via biomineralization during cultivation. Omics analysis revealed the upregulation of pyruvate metabolism and accumulation of DL-3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) in the presence of Sb(III).Using pyruvate as the sole carbon source in a chemically defined medium significantly enhanced Sb(III) biomineralization ratio from 20.8 % to 90.4 % compared with that using R2A medium. Additionally, reduced Sb(III) biomineralization and intracellular pH levels were observed following aceE gene knockout in Enterobacter sp. Z1. However, this impaired phenotype was rescued by complementing the aceE gene or introducing purified AceE into the bacterial lysates. Notably, AceE exhibited binding affinity for Sb(III). Our findings revealed the pyruvate–HMG-CoA pathway as the mechanism underlying Sb biomineralization, facilitating the release of Sb ions from tartrate and maintaining intracellular pH stability, thereby catalyzing Sb2O3 synthesis. This study provides insights into the Sb biogeochemical cycle.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


