Guanidinium chloride as multifunctional electrolyte additives for highly reversible aqueous zinc batteries
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Aqueous zinc-ion battery (ZIB) show promise for energy storage but face challenges in reversibility due to side reactions and dendrite growth, limiting practical application. Herein, we present a highly reversible aqueous ZIB where guanidinium chloride (GdmCl) assists the construction of protective solid-electrolyte interface (SEI), modifying the solvation structure of Zn2+, manipulate crystallographic orientation of Zn deposition. The Gdm+-based interface not only physically insulate free water in the electrolyte but also remove the reactive coordinated water molecules when hydrated Zn2+ pass through the interface. In addition, the Cl- from additive repel coordinated water molecules which further reduce the number active coordinated water molecules. Therefore, the side reactions were dramatically inhibited. Furthermore, Gdm+ facilitate the preferential growth of the Zn (002) crystal planes of Zn by blocking the (101) and (100) planes, inducing planar and dendrite-free deposition. As a result, dendrite-free Zn plating/stripping is achieved with a coulombic efficiency of ∼99.7 % over 300 cycles in Zn//Cu cells, stable charge/discharge performance for 1500 h in Zn//Zn cells, and an impressive cycle retention of 89 % over 1200 cycles in Zn//MnO2 cells. The additive also endows the electrolyte with antifreeze properties which enabling the cell to be operated at −20 °C, showing vast practical application potential
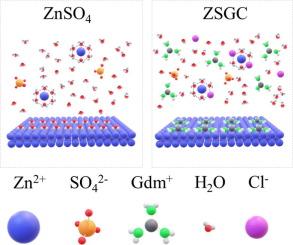
氯化胍作为高可逆水性锌电池的多功能电解质添加剂
水溶液锌离子电池(ZIB)在储能方面表现出良好的前景,但由于副反应和枝晶生长,其可逆性面临挑战,限制了其实际应用。在此,我们提出了一种高度可逆的水相ZIB,其中氯化胍(GdmCl)协助构建保护性固-电解质界面(SEI),改变Zn2+的溶剂化结构,操纵Zn沉积的晶体取向。基于Gdm+的界面不仅物理隔离了电解质中的自由水,而且当水合Zn2+通过界面时,还去除了反应性配位水分子。此外,来自添加剂的Cl-排斥配位水分子,进一步减少了活性配位水分子的数量。因此,副反应被显著抑制。此外,Gdm+通过阻断Zn的(101)和(100)晶面,促进Zn的Zn(002)晶面的优先生长,诱导平面和无枝晶沉积。结果表明,在Zn/ Cu电池中,无枝晶镀锌/剥离的库仑效率在300次循环中达到99.7 %,在Zn//Zn电池中,1500 h的充电/放电性能稳定,在Zn//MnO2电池中,在1200次循环中,循环保留率达到89 %。该添加剂还使电解质具有防冻特性,使电池能够在- 20 °C下工作,显示出巨大的实际应用潜力
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
文献相关原料
公司名称
产品信息
麦克林
Guanidinium chloride (GdmCl)
麦克林
Guanidinium chloride
阿拉丁
Manganese acetate (MnAc2)
阿拉丁
sodium sulfate (Na2SO4)
阿拉丁
zinc sulfate heptahydrate (ZnSO4·7H2O)
阿拉丁
Manganese acetate
阿拉丁
Sodium sulfate
阿拉丁
Zinc sulfate heptahydrate
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


