Design and operation optimization of a novel closed-loop NCM precursor resynthesis from spent LIB assisted by roasting and wastewater electrolysis
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
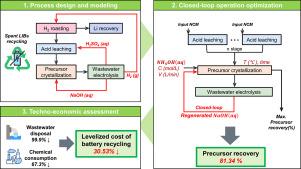
Recent studies on spent lithium-ion battery (LIB) recycling have focused on experimentally testing the direct resynthesis of NCM (NiCoMnO2) precursors from spent LIBs at a laboratory scale. However, the excessive use of chemicals, wastewater disposal, and operation optimization present issues in scaling to industrial applications. To resolve these challenges, this study proposes a novel closed-loop NCM precursor resynthesis assisted by roasting and wastewater electrolysis. The proposed system consists of three main processes: hydrogen roasting for lithium recovery, NCM precursor crystallization, and wastewater electrolysis for chemical regeneration. First, spent NCM cathodes were decomposed into metal oxides by hydrogen roasting, and water-soluble lithium oxide was separated from the metal oxide mixture. Second, the remaining metal oxide mixture was subjected to acid leaching, followed by NCM precursor crystallization. Finally, the wastewater generated from precursor crystallization was then treated using anion-exchange membrane-based electrolysis to regenerate the chemicals. The regenerated chemicals were reused in each process unit to create a closed-loop system. Furthermore, closed-loop operation optimization was conducted, leading to increased productivity and efficient time utilization. Under optimal operating conditions, the proposed system achieved a precursor recovery of 81.34%, which is 1.61% higher than that of the conventional system. Furthermore, economic analysis showed that the levelized cost of battery recycling of the proposed system was 30.53% lower than that of the conventional system. These results demonstrate that the proposed system could be a viable solution for sustainable closed-loop battery recycling by effectively reducing chemical consumption and wastewater disposal, while also improving productivity.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


