Mechanistic insights into the evolution of Cu active center in acetylene hydrochlorination
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Reserve–rich Cu–based catalysts are attractive for their favorable cost and sustainability and have exhibited extensive catalytic activities in the conversion of acetylene. However, the variable–valence and the presence of multi–species as well as the complexity of catalytic system pose challenges in deciphering the evolution process of Cu active center during working life–time. Herein, we investigated the evolution process of multivalent Cu–based species (Cu2+, Cu+ and Cu0) as model active centers for acetylene hydrochlorination. The reduction of Cu2+ driven by the activated carbon support and acetylene as well as oxidation of Cu0 induced by hydrogen chloride, have been clarified for these species, both of which with the terminated Cu+ species identified as the stable catalytic active center. Theoretical calculations have revealed the thermodynamics underlying the mechanism of species evolution determined by the covalent bond transition within Cu species, with comparisons of the differences in catalytic kinetics between sites. Moreover, a specific pathway for the catalytic decomposition of acetylene into coke deposits by Cu+ species was proposed. This knowledge provides mechanistic insights into the evolution process of Cu active centers in acetylene hydrochlorination, paving the way for understanding catalytic behavior and accurate catalyst design for new improved Cu–catalyzed ethynylation reactions.
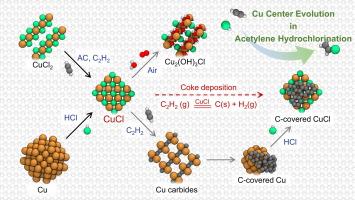

乙炔氢氯化反应中Cu活性中心演化的机理研究
储量丰富的铜基催化剂具有低廉的成本和可持续性,在乙炔转化中表现出广泛的催化活性。然而,铜的价态多变性、多组分的存在以及催化体系的复杂性,给破译铜活性中心在工作寿命期间的演化过程带来了挑战。在此,我们研究了多价Cu基物质(Cu2+, Cu+和Cu0)作为乙炔氢氯化反应的模型活性中心的演化过程。在活性炭载体和乙炔驱动下的Cu2+还原作用以及氯化氢诱导的Cu0氧化作用都得到了澄清,并确定了末端的Cu+为稳定的催化活性中心。理论计算揭示了Cu物种内部共价键转变决定物种进化机制的热力学基础,并比较了不同位点之间催化动力学的差异。此外,还提出了一种Cu+催化乙炔分解成焦炭的特定途径。这一知识为乙炔加氢氯化反应中Cu活性中心的演化过程提供了机理见解,为理解催化行为和准确设计新的改进Cu催化乙基化反应铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


