Discovery of the Salicylaldehyde-Based Compound DDO-02267 as a Lysine-Targeting Covalent Inhibitor of ALKBH5
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
N6-methyladenosine (m6A) is a crucial mRNA epigenetic modification in eukaryotes, and its methylation regulation is associated with the proliferation and metastasis of diverse tumor cells. ALKBH5 functions as a demethylase for m6A and plays a role in the demethylation process, thus influencing tumor cell growth and migration. However, there are limited reports on selective small molecule inhibitors of ALKBH5. Herein, we designed and synthesized the ALKBH5 covalent inhibitor DDO-02267 by analyzing the protein structure of ALKBH5 and introducing salicylaldehyde warhead into noncovalent small molecule ligand. DDO-02267 specifically targeted Lys132 within ALKBH5, demonstrating significant selectivity for ALKBH5 in vitro. Additionally, DDO-02267 increased m6A levels and targeted the ALKBH5-AXL signaling axis in AML cells. The compound DDO-02267 can serve as a probe for investigating the biological function of mRNA demethylase and may inspire the development of future ALKBH5 inhibitors.
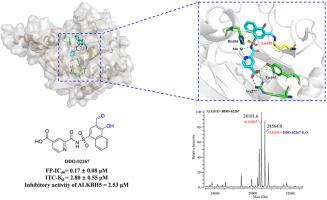
水杨醛基化合物DDO-02267作为赖氨酸靶向ALKBH5共价抑制剂的发现
n6 -甲基腺苷(m6A)是真核生物中一种重要的mRNA表观遗传修饰,其甲基化调控与多种肿瘤细胞的增殖和转移有关。ALKBH5作为m6A的去甲基化酶,在去甲基化过程中发挥作用,从而影响肿瘤细胞的生长和迁移。然而,关于ALKBH5选择性小分子抑制剂的报道有限。本文通过对ALKBH5蛋白结构的分析,将水杨醛战斗部引入非共价小分子配体中,设计合成了ALKBH5共价抑制剂ado -02267。DDO-02267特异性靶向ALKBH5中的Lys132,对ALKBH5显示出显著的体外选择性。此外,DDO-02267增加了AML细胞中的m6A水平并靶向ALKBH5-AXL信号轴。化合物DDO-02267可作为研究mRNA去甲基化酶生物学功能的探针,并可能启发未来ALKBH5抑制剂的开发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


