Nitrogen-doped carbon decorated with metal carbide and metal in Li-S chemistry up to 3500 cycles
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
In pursuit of the new generation commercial batteries, lithium-sulfur batteries (LSBs) put up great feasibility. However, as a consequence of slow redox kinetics for lithium polysulfides (LiPSs) and presence of lithium dendrites, the commercialization processes have been seriously hindered. These bottleneck problems can be solved by modifying the polypropylene (PP) separator. The present study focuses on a design of Co/Mo2C/nitrogen-doped carbon (Co/Mo2C/NC) composite with a hollow hexahedron heterostructure, which serves as an anchor agent for immobilizing LiPSs and accelerating the redox reactions. Co reinforcing the absorbency to LiPSs is revealed by density functional theory (DFT) calculations. Mo2C enhances the absorbency to LiPSs and picks up speed of transport for lithium-ion. Additionally, NC exhibits the remarkable catalytic performance for LiPSs. Through the synergic action of Co, Mo2C, and NC, the catalytical efficiency is significantly improved for conversion of LiPSs. Therefore, an enhanced PP separator (named as Co/Mo2C/NC-PP) is prepared to hold back the shuttle of LiPSs. On the side, the experiment certifies that the Co/Mo2C/NC-PP separator assembled lithium-sulfur battery (LSB) shows a favorable electrochemical performance. At 0.5C, the LSB brings out 1268 mAh/g for the first discharge capacity and capacity retention rate is up to 79 % after 100 cycles. In the meantime, the original discharge capacity has 992 mAh/g at 1C, each cycle only experiences a minor attenuation of 0.013 % during 3500 cycles as well. Even if the areal sulfur loading is 6.0mg cm−2 and 2.5μL mg−1 for lean electrolyte/sulfur (E/S) ratio, a good capacity retention is still kept at 0.2C. This strategy opens up a fresh way for efficient design of separator modification material for high-performance LSBs.
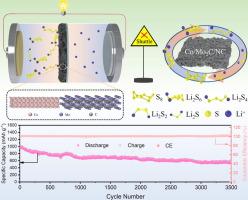
以金属碳化物和金属装饰的氮掺杂碳在Li-S化学中可循环达3500次
在追求新一代商用电池的过程中,锂硫电池呈现出极大的可行性。然而,由于锂多硫化物(LiPSs)的氧化还原动力学缓慢和锂枝晶的存在,严重阻碍了商业化进程。这些瓶颈问题可以通过对聚丙烯(PP)分离器进行改造来解决。设计了一种具有中空六面体异质结构的Co/Mo2C/氮掺杂碳(Co/Mo2C/NC)复合材料,作为固定化LiPSs和加速氧化还原反应的锚定剂。通过密度泛函理论(DFT)计算揭示了Co对LiPSs的吸收增强作用。Mo2C增强了对LiPSs的吸收,加快了锂离子的输运速度。此外,NC对LiPSs具有显著的催化性能。通过Co、Mo2C和NC的协同作用,显著提高了LiPSs转化的催化效率。因此,制备了一种增强型PP分离器(Co/Mo2C/NC-PP),以阻挡LiPSs的穿梭。另一方面,实验证明了Co/Mo2C/NC-PP分离器组装的锂硫电池(LSB)具有良好的电化学性能。在0.5C时,LSB第一次放电容量为1268 mAh/g, 100次循环后容量保持率高达79 %。同时,在1C下,原始放电容量为992 mAh/g,在3500次循环中,每个循环只经历0.013 %的轻微衰减。即使面积硫负荷为6.0mg cm−2,贫电解质/硫(E/S)比为2.5μL mg−1,在0.2C时仍能保持良好的容量保持。该策略为高效设计高性能lsb分离器改性材料开辟了一条新途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


