Screen-printed electrodes covered by mercury film or meniscus
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
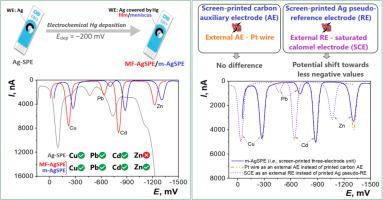
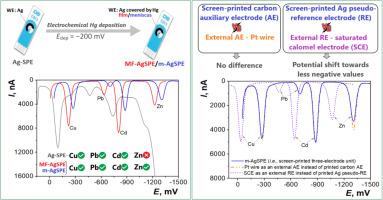
For the first time, silver screen-printed electrodes covered by mercury film (MF-AgSPE) and mercury meniscus (m-AgSPE) were designed, prepared and tested. The precise electrochemical preparation procedure for mercury deposition at the silver working electrode (WE) surface of commercial AgSPE was used. A simple and quick way to prepare m-AgSPE is transferring a precisely defined mercury drop from a hanging mercury drop electrode (HMDE) at WE. Deposited mercury dissolves silver and it gradually converts into solid or paste amalgam. The potential of hydrogen evolution with these electrodes has maximum values in aqueous solutions and is comparable to that on mercury electrodes. The 20 µm thick mercury film allows working with the liquid surface during the day and its deposition takes approximately 15 min. Testing of MF- and m-AgSPEs was performed by voltammetric methods with and without accumulation of analytes on the electrode, in a batch electrode arrangement and also in a flow-through system. For comparison, measurements were carried out with printed solid amalgam electrodes, HMDE, and also with the original commercial AgSPE. Simultaneous measurement of zinc, cadmium, lead, and copper cations on m-AgSPE by stripping voltammetry allowed to determine their concentrations <1 µg L-1. Comparison of measurements with the printed pseudo-reference electrode of SPE and an external calomel electrode showed that the differences in the peak potentials can be up to 200 mV. Commercial large-scale production of printed electrodes makes them a practically unlimited source for the rapid preparation of inexpensive electrodes with an ideally smooth liquid surface.
由水银薄膜或半月板覆盖的丝网印刷电极
首次设计、制备和测试了汞膜覆盖银丝网印刷电极(MF-AgSPE)和汞半月板覆盖银丝网印刷电极(m-AgSPE)。采用精密电化学工艺,在商用AgSPE的银工作电极(WE)表面沉积汞。制备m-AgSPE的一种简单而快速的方法是从WE的悬挂汞滴电极(HMDE)转移精确定义的汞滴。沉积的汞溶解银,并逐渐转化为固体或糊状汞合金。这些电极的析氢电位在水溶液中具有最大值,与汞电极相当。20微米厚的汞膜可以在白天与液体表面一起工作,其沉积大约需要15分钟。通过伏安法对MF-和m- agspe进行测试,在电极上有和没有分析物积累,在批电极布置中,也在流式系统中。为了进行比较,使用印刷固体汞合金电极HMDE和原始商用AgSPE进行了测量。通过溶出伏安法在m-AgSPE上同时测量锌,镉,铅和铜阳离子,可以确定它们的浓度小于1 μ g L-1。与印刷的SPE伪参比电极和外置甘汞电极的测量结果比较,峰电位的差异可达200 mV。印刷电极的商业大规模生产使它们成为快速制备具有理想光滑液体表面的廉价电极的几乎无限来源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


