Thermodynamic Modeling of Phase Equilibrium of CO2 + TBPB + THF + NaCl + Water System
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
In this work, the effect of adding thermodynamic promoters tetra-n-butyl phosphonium bromide (TBPB) and tetrahydrofuran (THF) on the conditions of CO2 gas hydrate formation is investigated. Considering that seawater is used for the formation of hydrate in large volume, the effect of NaCl as the dominant salt in saline water on the phase equilibrium of the CO2 + TBPB system in the presence of THF is studied. Also, in order to predict the conditions of hydrate formation, the van der Waals–Plateau model has been used to model the hydrate phase equilibrium. The SRK equation of state has been used to check the gas phase. The e-NRTL model has been used to estimate the activity coefficient, and the model parameters for the system are presented under study. The results show that addition of NaCl (3% mass fraction) to the (TBPB + THF) aqueous solution changes the equilibrium conditions of CO2 hydrate formation. It is observed that the hydrate pressure predicted by the provided model is in good agreement with the available experimental data on the CO2 hydrate phase equilibrium in the presence of TBPB, THF, and NaCl. The model parameters are also estimated by using experimental data and the optimization method. The average absolute deviation for hydrate dissociation pressure is 3.23%.
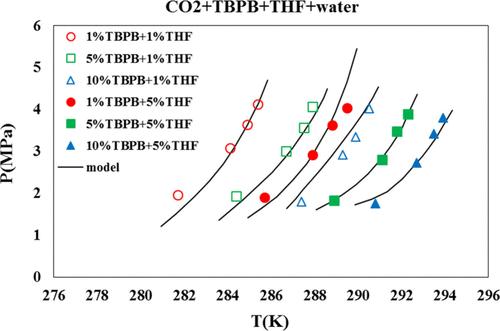
CO2 + TBPB + THF + NaCl +水体系相平衡的热力学模拟
研究了热力学促进剂四正丁基溴化磷(TBPB)和四氢呋喃(THF)的加入对CO2气体水合物生成条件的影响。考虑到海水是大体积生成水合物的介质,研究了在THF存在下,NaCl作为咸水中的优势盐对CO2 + TBPB体系相平衡的影响。此外,为了预测水合物的形成条件,还采用了范德华斯-高原模型来模拟水合物的相平衡。用SRK状态方程来检验气相。利用e-NRTL模型对活度系数进行了估计,并给出了系统的模型参数。结果表明:在(TBPB + THF)水溶液中加入3%质量分数的NaCl改变了CO2水合物生成的平衡条件;结果表明,该模型预测的水合物压力与已有的TBPB、THF和NaCl存在下CO2水合物相平衡的实验数据吻合较好。利用实验数据和优化方法对模型参数进行了估计。水合物解离压力的平均绝对偏差为3.23%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


