Pyrimidine synthesis enzyme CTP synthetase 1 suppresses antiviral interferon induction by deamidating IRF3
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Metabolism is typically contextualized in conjunction with proliferation and growth. The roles of metabolic enzymes beyond metabolism—such as in innate immune responses—are underexplored. Using a focused short hairpin RNA (shRNA)-mediated screen, we identified CTP synthetase 1 (CTPS1), a rate-limiting enzyme of pyrimidine synthesis, as a negative regulator of interferon induction. Mechanistically, CTPS1 interacts with and deamidates interferon regulatory factor 3 (IRF3). Deamidation at N85 impairs IRF3 binding to promoters containing IRF3-responsive elements, thus muting interferon (IFN) induction. Employing CTPS1 conditional deletion and IRF3 deamidated or deamidation-resistant knockin mice, we demonstrated that CTPS1-driven IRF3 deamidation restricts IFN induction in response to viral infection in vivo. However, during immune activation, IRF3 deamidation by CTPS1 is inhibited by glycogen synthase kinase 3 beta (GSK3β) to promote IFN induction. This work demonstrates how CTPS1 tames innate immunity independent of its role in pyrimidine synthesis, thus expanding the functional repertoire of metabolic enzymes into immune regulation.
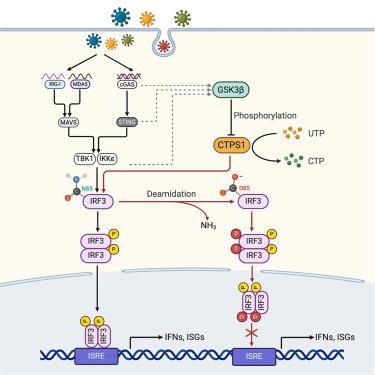
嘧啶合成酶CTP合成酶1通过去酰胺IRF3抑制抗病毒干扰素诱导
代谢通常与增殖和生长结合在一起。代谢酶在新陈代谢之外的作用——比如先天免疫反应——还没有得到充分的探索。通过聚焦短发夹RNA (shRNA)介导的筛选,我们鉴定出嘧啶合成的限速酶CTP合成酶1 (CTPS1)是干扰素诱导的负调节因子。在机制上,CTPS1与干扰素调节因子3 (IRF3)相互作用并脱酰胺。N85处的脱酰胺会损害IRF3与含有IRF3响应元件的启动子的结合,从而抑制干扰素(IFN)的诱导。利用CTPS1条件缺失和IRF3脱酰胺或抗脱酰胺敲入小鼠,我们证明了CTPS1驱动的IRF3脱酰胺在体内对病毒感染的反应中限制了IFN的诱导。然而,在免疫激活过程中,糖原合成酶激酶3β (GSK3β)抑制CTPS1对IRF3的脱酰胺作用,从而促进IFN的诱导。这项工作证明了CTPS1如何独立于其在嘧啶合成中的作用来驯化先天免疫,从而将代谢酶的功能库扩展到免疫调节。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


