Functional Group Effects on the Interfacial Adsorption of Arylquinoline-3-Carbonitriles on Iron: A DFT-D3 Investigation of Surface Interaction Mechanisms
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
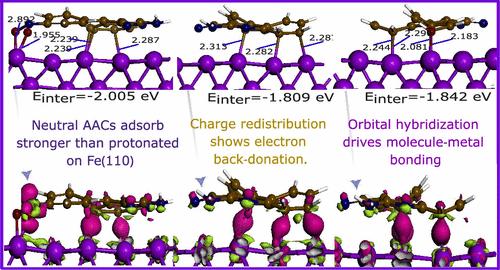
Reliable corrosion inhibition systems are crucial for extending the lifespan of industrial metal structures. Quinolines, with their high adsorption capacity and protective efficiency, are promising next-generation inhibitors. However, the impact of substitutions on their coordination with iron surfaces requires deeper understanding. Herein, we investigate the influence of various functional groups on the adsorption behavior of three 2-amino-4-arylquinoline-3-carbonitriles (AACs) on iron surfaces using first-principles density functional theory calculations. Results reveal that nitrophenyl and hydroxyphenyl significantly enhance the adsorption strength of AACs on the Fe(110) surface, facilitated by donor–acceptor interactions. Neutral molecules were more stable than their protonated counterparts. Key results show strong adsorption energies, with values ranging from −2.005 to −1.809 eV for the AACs, along with significant electron gains across carbon atoms as indicated by Bader charge analysis. These strong interactions result in notable charge redistribution and bond formation, as shown by projected density of states and electron density difference iso-surfaces. Furthermore, electron localization function analysis indicates that van der Waals interactions, influenced by multiple nitrogen atoms, play a crucial role in stabilizing the adsorbed molecules. Stronger adsorption through electron donation and retro-donation mechanisms suggests enhanced corrosion protection efficiency of these substituted quinolines. The conductor-like screening model for real solvents analysis provides complementary insights into the solvation characteristics. Overall, the findings demonstrate the specific role functional groups play in the coordination of arylquinoline-3-carbonitriles with iron surfaces.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


