Binding of Bioactive Ammonium Ions in Water with a Cavity-Based Selectivity: Water Solubilization versus Micellar Incorporation
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Many bioactive molecules contain primary ammonium groups, generating significant interest in developing selective receptors for ammonium ions. A promising strategy involves the use of polyaromatic cavitands to achieve size and shape selectivity through their cavity. However, designing effective receptors for ammonium ions in aqueous media is challenging due to the competitive nature of water. Calix[5]arenes are known to selectively bind primary ammonium ions over secondary, tertiary, and quaternary ammonium ions in organic solvents. Here, we report on the binding properties of a calix[5]arene, which bears carboxyl groups on its small rim, in organic solvents and aqueous media. This receptor was transferred in water either through deprotonation of its carboxyl groups or by incorporation into dodecylphosphocholine micelles. 1H Nuclear Magnetic Resonance data confirmed the endo complexation of various primary ammonium ions in not only organic solvents but also both aqueous media. Cavity-based selectivity was also observed, validating the cavitand strategy for the selective binding of ammonium ions in water. Unique binding properties, driven by the calix[5]arene’s intrinsic recognition ability and the hydrophobic effect, were observed in water. Notably, binding affinities for dopamine and lysine derivatives with log Ka values of >3.9 were determined. The direct solubilization of the receptor outperformed micellar incorporation due to the hydrophilic nature of the primary ammonium ions, which hinders their uptake into micelles. These results offer promising perspectives for the development of efficient chemosensors for the characterization of bioactive ammonium ions in water.
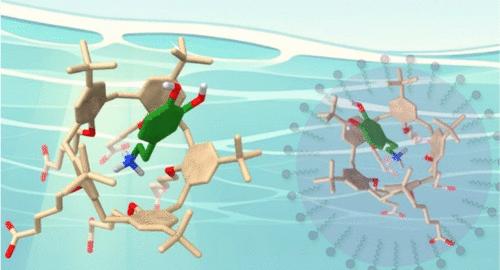
基于空腔选择性的生物活性铵离子在水中的结合:水的溶解与胶束的结合
许多具有生物活性的分子都含有伯胺基团,这使得人们对开发选择性铵离子受体产生了极大的兴趣。一种很有前途的策略是使用多芳族空腔体来通过它们的空腔实现尺寸和形状的选择性。然而,由于水的竞争性,在水介质中设计有效的铵离子受体是具有挑战性的。杯状芳烃在有机溶剂中选择性地结合伯铵离子而不是仲、叔、季铵离子。本文报道了一种杯状[5]芳烃在有机溶剂和水介质中的结合特性,它的小边缘上有羧基。该受体通过其羧基的去质子化或结合到十二烷基磷胆碱胶束中在水中转移。1H核磁共振数据证实了各种伯胺离子不仅在有机溶剂中,而且在两种水介质中都有内位络合作用。基于空腔的选择性也被观察到,验证了在水中铵离子选择性结合的空腔策略。在水中观察到独特的结合性能,这是由杯状[5]芳烃的固有识别能力和疏水效应驱动的。值得注意的是,多巴胺和赖氨酸衍生物与log Ka值为>;3.9的结合亲和力被确定。由于伯胺离子的亲水性,受体的直接增溶优于胶束掺入,这阻碍了它们被胶束吸收。这些结果为开发用于表征水中生物活性铵离子的高效化学传感器提供了有希望的前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


