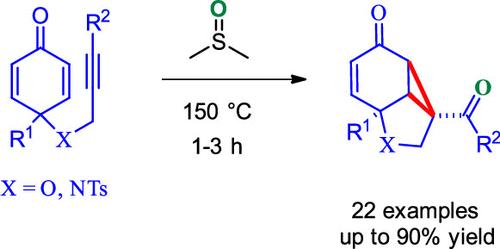
Reaction of 4-(Alkynyloxy)cyclohexa-2,5-dienones with Dimethyl Sulfoxide: A Catalyst-Free Formation of 6/5/3-Fused Tricyclic Enones
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
6/5/3-Fused tricyclic enones were obtained when 4-(alkynyloxy)cyclohexa-2,5-dienones were treated with DMSO at 150 °C. The reaction proceeded via a four-membered oxathietene intermediate. The protocol developed is atom economical, has a broad substrate scope, and is amenable to gram-scale synthesis. The products obtained are susceptible to further synthetic transformations.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


