Development of Thermosensitive Mucoadhesive Gel Based Encapsulated Lipid Microspheres as Nose-to-Brain Rivastigmine Delivery System
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Alzheimer’s disease (ALZ) is a neurodegenerative disease that damages neuronal cells and causes decline in cognitive abilities. Administration of cholinesterase inhibitor compounds is the primary choice in the treatment of ALZ, one of which is rivastigmine (RVT). Several routes of administration of RVT are available, such as oral and transdermal. However, in the oral route, RVT has low bioavailability, undergoes first-pass metabolism, and the presence of the blood-brain barrier (BBB) reduces the therapeutic concentration of RVT. The transdermal route is nonselective target in the brain. This study aims to combine thermosensitive mucoadhesive gel (TG) and lipid microspheres (LM) as a drug delivery system to improve the efficacy of RVT. Combining these will prevent systemic side effects of RVT and increase drug concentration in the brain. LM was formulated with varying concentrations of Compritol polymer. The results of LM evaluation showed the values of particle size, PDI, and %EE and %DL were 8.519 μm, 0.018 ± 0.004, 72.54%, and 76.43%, respectively. The TG formulation can provide a liquid form at room temperature (25 °C) and a gel at nasal temperature (35 °C). Hemolytic and HET-CAM tests confirmed TG RVT LM’s safety for use. Ex vivo studies showed controlled and sustained release of TG RVT LM, and in vivo studies showed TG RVT LM a higher pharmacokinetic profile in the brain than oral formulations and injections. The Cmax was found to be 7.05 ± 0.55 μg/cm3, Tmax was 24 h, and AUC0–24, which is related to the effectiveness of brain targeting, was 225.73 μg/cm3. In conclusion, this study shows the successful development of TG RVT LM, as evidenced by improved drug delivery to the brain, which is characterized by higher concentrations of RVT in the brain compared with oral and injectable RVT, this delivery system shows potential as a future treatment for Alzheimer’s disease.
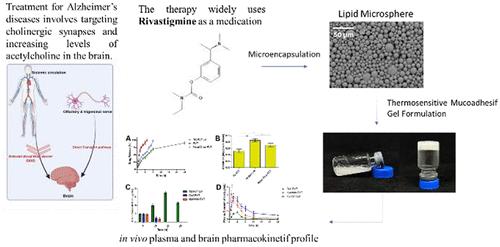
热敏粘粘凝胶基脂质微球鼻脑给药系统的研制
阿尔茨海默病(ALZ)是一种神经退行性疾病,损害神经元细胞并导致认知能力下降。胆碱酯酶抑制剂化合物是治疗ALZ的主要选择,其中之一是利瓦斯汀(RVT)。有几种给药途径,如口服和透皮给药。然而,在口服途径中,RVT具有低生物利用度,经历首过代谢,血脑屏障(BBB)的存在降低了RVT的治疗浓度。透皮途径是脑内的非选择性靶点。本研究旨在将热敏黏附凝胶(TG)与脂质微球(LM)结合作为一种给药系统来提高RVT的疗效。结合这些药物可以防止RVT的全身副作用,并增加大脑中的药物浓度。用不同浓度的康普利醇聚合物配制LM。LM评价结果显示,样品的粒径、PDI、%EE和%DL分别为8.519 μm、0.018±0.004、72.54%和76.43%。TG制剂可在室温(25℃)下提供液体形式,在鼻温(35℃)下提供凝胶形式。溶血和热成像- cam测试证实TG RVT LM使用安全。体外研究显示TG RVT LM具有可控和持续的释放,体内研究显示TG RVT LM在大脑中的药代动力学特征高于口服制剂和注射剂。Cmax为7.05±0.55 μg/cm3, Tmax为24 h,与脑靶向效果相关的AUC0-24为225.73 μg/cm3。总之,本研究表明TG RVT LM的成功开发,通过改善药物给药到大脑,其特点是与口服和注射RVT相比,RVT在大脑中的浓度更高,这种给药系统在未来治疗阿尔茨海默病方面具有潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


