Impact of Potassium Doping on a Two-Dimensional Kagome Organic Framework on Ag(111)
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
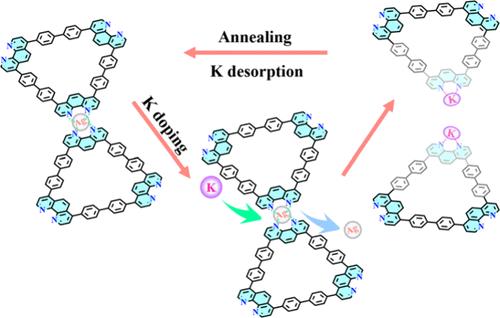
Alkali element doping has significant physical implications for two-dimensional materials, primarily by tuning the electronic structure and carrier concentration. It can enhance interface electronic interactions, providing opportunities for effective charge transfer at metal–organic interfaces. In this work, we investigated the effects of gradually increasing the level of K doping on the lattice structure and electronic properties of an organometallic coordinated Kagome lattice on a Ag(111) surface. With the introduction of K dopants into the 4-fold N–Ag coordinated Kagome lattice, the highly periodic Kagome lattice gradually tends to become discrete. Combining synchrotron radiation photoemission spectroscopy, scanning tunneling microscopy/spectroscopy, and density functional theory calculations, we revealed the mechanism of structural transformation of the lattice, i.e., the change in thermodynamically favored structures caused by competition of electron donors. As an electron donor with a lower ionization energy, K adatoms tend to replace the Ag adatoms and form a more thermodynamically stable N–K coordination structure. Moreover, enhanced charge transfer from K to the Kagome lattice induced a rigid shift of the Fermi level. Our investigation provides new insights for the study of alkali-doped organometallic nanostructures.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


