Discovery of MDI-114215: A Potent and Selective LIMK Inhibitor To Treat Fragile X Syndrome
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
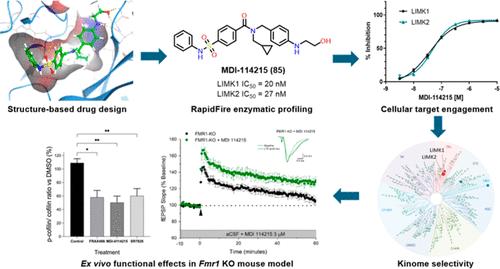
LIMKs are serine/threonine and tyrosine kinases responsible for controlling cytoskeletal dynamics as key regulators of actin stability, ensuring synaptic health through normal synaptic bouton structure and function. However, LIMK1 overactivation results in abnormal dendritic synaptic development that characterizes the pathogenesis of Fragile X Syndrome (FXS). As a result, the development of LIMK inhibitors represents an emerging disease-modifying therapeutic approach for FXS. We report the discovery of MDI-114215 (85), a novel, potent allosteric dual-LIMK1/2 inhibitor that demonstrates exquisite kinome selectivity. 85 reduces phospho-cofilin in mouse brain slices and rescues impaired hippocampal long-term potentiation in brain slices from FXS mice. We also show that LIMK inhibitors are effective in reducing phospho-cofilin levels in iPSC neurons derived from FXS patients, demonstrating 85 to be a potential therapeutic candidate for FXS that could have broad application to neurological disorders or cancers caused by LIMK1/2 overactivation and actin instability.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


