Development of [68Ga]Ga/[177Lu]Lu-DOTA-NI-FAPI-04 Containing a Nitroimidazole Moiety as New FAPI Radiotracers with Improved Tumor Uptake and Retention
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Fibroblast activation protein (FAP), which is overexpressed in cancer-associated fibroblasts (CAFs), represents a promising target for cancer diagnosis and therapy. Hypoxia is a common feature of solid tumors. A bivalent agent, DOTA-NI-FAPI-04 (1), was developed by incorporating hypoxia-sensitive nitroimidazole (NI) into the FAP-targeting agent FAPI-04. Compound 1 exhibited a strong FAP binding affinity with an IC50 of 7.44 nM. Radiolabeled [68Ga]Ga-1 and [177Lu]Lu-1 demonstrated enhanced in vitro cell uptake. In vivo positron emission tomography/computed tomography (PET/CT) imaging showed that [68Ga]Ga-1 displayed significantly higher specific uptake and retention in U87MG tumor-bearing mice compared to [68Ga]Ga-FAPI-04 (SUVavg: 7.87 vs 1.99% ID/mL at 120 min). Biodistribution studies confirmed superior tumor uptake of [68Ga]Ga-1 (48.15 vs 5.72% ID/g at 120 min). Similarly, [177Lu]Lu-1 exhibited higher tumor uptake than [177Lu]Lu-FAPI-04 (50.75 vs 20.48% ID/g at 120 min). These preliminary results suggest that a nitroimidazole-containing bivalent-targeting agent, [68Ga]Ga/[177Lu]Lu-1, is a promising candidate for tumor theranostics.
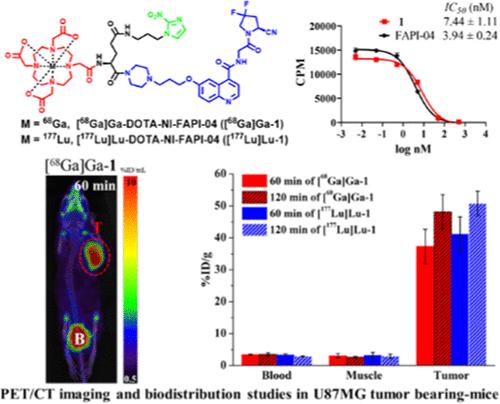
含有硝基咪唑基团的[68Ga]Ga/[177Lu]Lu-DOTA-NI-FAPI-04新型FAPI放射性示踪剂的研制
成纤维细胞激活蛋白(FAP)在癌症相关成纤维细胞(CAFs)中过表达,是癌症诊断和治疗的一个有希望的靶点。缺氧是实体瘤的共同特征。将对缺氧敏感的硝基咪唑(NI)掺入FAPI-04中,制备了一种双价药物DOTA-NI-FAPI-04(1)。化合物1具有较强的FAP结合亲和力,IC50为7.44 nM。放射性标记的[68Ga]Ga-1和[177Lu]Lu-1增强了体外细胞摄取。体内正电子发射断层扫描/计算机断层扫描(PET/CT)成像显示,与[68Ga]Ga-FAPI-04相比,[68Ga]Ga-1在U87MG荷瘤小鼠中的特异性摄取和保留率显著提高(120 min时SUVavg: 7.87 vs 1.99% ID/mL)。生物分布研究证实,[68Ga]Ga-1在120分钟时的肿瘤摄取优势(48.15 vs 5.72% ID/g)。同样,[177Lu]Lu-1比[177Lu]Lu-FAPI-04表现出更高的肿瘤摄取(120min时50.75 vs 20.48% ID/g)。这些初步结果表明,含硝基咪唑的二价靶向药物[68Ga]Ga/[177Lu]Lu-1是一种很有前景的肿瘤治疗药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


