The Alkylation of Phenol with Methanol: The Influence of Acid–Base Properties of X Zeolite on the Selectivity of para- and meta-Cresol
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The vapor-phase alkylation of phenol with methanol was investigated on X zeolites and modified X zeolites. First, the difference of product distribution was tested between acid zeolite (HZSM-5, HX, HMCM-22, and Hβ) and basic zeolite X (KX and CsX). Then, X zeolites were modified with Li, K, Cs, Ca, Mg, La, and Ce ion exchange to adjust the acid–base properties of the zeolites. Finally, the type of acid sites and strength of acid–base sites on zeolite catalysts were determined by characterization techniques such as Py-IR and TPD, and the alkylation of phenol with methanol was tested. The results showed that O-alkylation products convert to the C-alkylation products on the B acid sites, which enhances the isomerization reaction, thereby increasing the proportion of meta-cresol in cresol. The results of TPD and IR indicated that weak basic sites on the zeolite promote the vertical adsorption of the aromatic ring and strong basic sites promote side-chain activation, while acid sites determine whether ring substitution or side-chain substitution occurs. During the reaction of phenol with methanol, the phenolic hydroxyl group strongly interacts with the surface of zeolites, leading to differences in the adsorption mode of the aromatic ring (vertical or parallel), which, in turn, alters the position of alkyl substitution. It is found that the proper acid–base property of X zeolites can selectively determine the desired alkylated products.
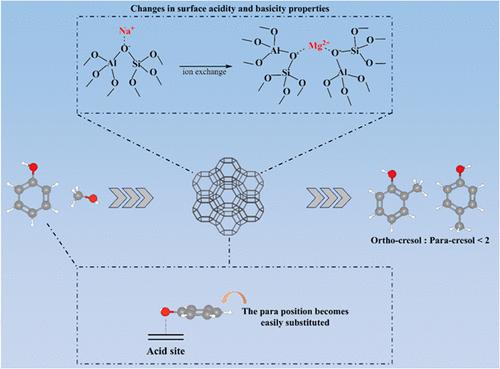
苯酚与甲醇的烷基化反应:X沸石的酸碱性质对对甲酚和间甲酚选择性的影响
研究了苯酚与甲醇在X沸石和改性X沸石上的气相烷基化反应。首先,比较了酸性沸石(HZSM-5、HX、HMCM-22和Hβ)与碱性沸石X (KX和CsX)的产物分布差异。然后用Li、K、Cs、Ca、Mg、La、Ce离子交换剂对X分子筛进行改性,调整分子筛的酸碱性质。最后,通过Py-IR和TPD等表征技术确定了沸石催化剂上酸位的类型和酸碱位的强度,并对苯酚与甲醇的烷基化反应进行了测试。结果表明,o -烷基化产物在B酸位点转化为c -烷基化产物,增强了异构化反应,从而增加了间甲酚在甲酚中的比例。TPD和IR结果表明,沸石上的弱碱性位点促进了芳环的垂直吸附,强碱性位点促进了侧链的活化,而酸性位点决定了是否发生环取代或侧链取代。在苯酚与甲醇的反应过程中,酚羟基与沸石表面发生强烈的相互作用,导致芳香环的吸附方式(垂直或平行)不同,进而改变烷基取代的位置。研究发现,适宜的X分子筛酸碱性质可以选择性地决定所需的烷基化产物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


