Cross-Dehydrogenative Coupling of Secondary Amines with Silanes Catalyzed by Agostic Iridium-NSi Species
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
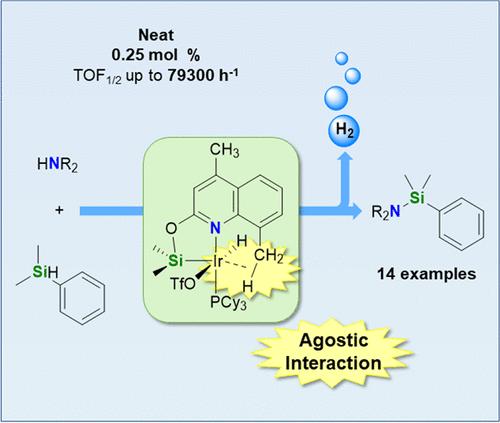
An active catalytic system for the cross-dehydrogenative coupling (CDC) of a wide range of secondary amines with silanes is reported. The iridium(III) derivatives [Ir(H)(X)(κ2-NSiDMQ)(L)] (NSiDMQ = {4,8-dimethylquinoline-2-yloxy}dimethylsilyl; L = coe, X = Cl, 2; L = coe, X = OTf, 3; L = PCy3, X = Cl, 4; L = PCy3, X = OTf, 5), which are stabilized by a weak yet noticeable Ir···H–C agostic interaction between the iridium and one of the C–H bonds of the 8-Me substituent of the NSiDMQ ligand, have been prepared and fully characterized. These species have proven to be effective catalysts for the CDC of secondary amines with hydrosilanes. The best catalytic performance (TOF1/2 = 79,300 h–1) was obtained using 5 (0.25 mol %), N-methylaniline, and HSiMe2Ph. The catalytic activity of the species [Ir(H)(OTf)(κ2-NSiQ)(PCy3)] (10, NSiQ = {quinoline-2-yloxy}dimethylsilyl) and [Ir(H)(OTf)(κ2-NSiMQ)(PCy3)] (11, NSiMQ = {4-methylquinoline-2-yloxy}dimethylsilyl), related to 5 but lacking the 8-Me substituent, is markedly lower than that found for 5. This fact highlights the crucial role of the 8-Me substituent of the NSiDMQ ligand in enhancing the catalytic performance of these iridium complexes.
无机铱- nsi催化仲胺与硅烷的交叉脱氢偶联
报道了一种用于多种仲胺与硅烷交叉脱氢偶联反应的活性催化体系。铱(III)衍生物[Ir(H)(X)(κ2-NSiDMQ)(L)] (NSiDMQ ={4,8-二甲基喹啉-2-氧基}二甲基硅基;L = coe, X = Cl, 2;L = coe, X = OTf, 3;L = PCy3, X = Cl, 4;L = PCy3, X = OTf, 5)是由铱与NSiDMQ配体8-Me取代基的一个C-H键之间微弱但明显的Ir···H-C相互作用稳定的。这些物种已被证明是仲胺与氢硅烷的有效催化剂。5 (0.25 mol %)、n -甲基苯胺和HSiMe2Ph的催化性能最佳(TOF1/2 = 79,300 h-1)。与5相关但缺少8-Me取代基的[Ir(H)(OTf)(κ2-NSiQ)(PCy3)] (10, NSiQ ={喹啉-2-基氧基}二甲基硅基)和[Ir(H)(OTf)(κ2-NSiMQ)(PCy3)] (11, NSiMQ ={4-甲基喹啉-2-基氧基}二甲基硅基)的催化活性明显低于5。这一事实突出了NSiDMQ配体的8-Me取代基在提高这些铱配合物的催化性能方面的关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


