Cisplatin/Apo-Transferrin Adduct: X-ray Structure and Binding to the Transferrin Receptor 1
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
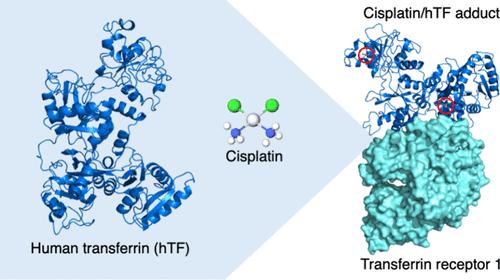
Here, we report the X-ray structure of the adduct formed upon reaction of cisplatin, one of the most prescribed anticancer agents for the clinic treatment of solid tumors, with the apo-form of human serum transferrin (hTF). Two Pt binding sites were identified in both molecules of the adduct present in the crystal asymmetric unit: Pt binds close to the side chains of Met256 and Met499 at the N- and C-lobe, respectively. In the crystal structure, the cisplatin moiety bound to Met256 also interacts with Ser616 from a symmetry related molecule. Structural analyses, together with in solution data, demonstrate that the presence of iron does not affect the ability of hTF to bind cisplatin and that the cisplatin binding does not significantly alter the overall conformation of the different forms of the protein that remain able to form a complex with the transferrin receptor 1 (TfR1). These data suggest that the different hTF forms can be used as nanocarriers for targeted (combined) metallodrug delivery.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


